|
Case Report
Primary malignant melanoma of the vagina: A rare and aggressive disease
1 Department of Gynecology and Obstetrics, Kantonsspital Luzern, Spitalstrasse, 6000 Luzern 16, Switzerland
Address correspondence to:
Barbara Kipp
Department of Gynecology and Obstetrics, Division of Gynecologic Oncology, Kantonsspital Luzern, Spitalstrasse, 6000 Luzern 16,
Switzerland
Message to Corresponding Author
Article ID: 100061Z08BK2020
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Kipp B, Christmann C. Primary malignant melanoma of the vagina: A rare and aggressive disease. J Case Rep Images Obstet Gynecol 2020;6: 100061Z08BK2020.ABSTRACT
Introduction: A primary malignant melanoma of the vagina (PMMV) is a rare and aggressive disease. At the time of diagnosis the tumors are mostly already large in size thus entailing a high risk for local recurrence and distant metastasis. Various treatment regimens such as local wide excision, radical surgical procedures, and nonsurgical treatment (primary radiotherapy, chemotherapy, and immunotherapy) have been described in literature. A standard treatment protocol has not yet been defined.
Case Report: We present an 89-year-old woman with abnormal vaginal bleeding, resulting in a malignant melanoma of the vagina. The tumor size revealed in magnetic resonance imaging (MRI) scan was 6.6 × 3.2 cm with close localization to the rectal wall. We recommended radical surgery to achieve free margins which she refused. Despite good local response after primary radiotherapy she died six months later because of distant metastasis.
Conclusion: In PMMV primary radiotherapy is a valuable treatment option whenever surgery is not feasible or refused by the patient. As we described in our case local control is achievable but the life limiting risk of distant metastasis remains. Further investigation to define a standard treatment protocol is needed.
Keywords: Malignant melanoma, Primary radiotherapy, Standard of treatment, Vagina
Introduction
Primary malignant melanoma of the vagina (PMMV) is a rare tumor entity which accounts for less than 1% of all melanomas and 1–5% of all malignant vaginal tumors. Only 500 cases have been reported in literature so far and long-term follow-up as well as pooled data is missing [1].
The reported 5-year overall survival (OS) of women with PMMV is poor, ranging from 13–32% and the major issues are the high risk for local recurrence and lymphatic as well as distant metastasis [2]. Predominantly postmenopausal women are affected. The typical location of tumor manifestation is the lower third of the vagina in the anterior vaginal wall [2]. At the time of diagnosis most tumors have a large diameter and size, resulting in a poor prognosis and overall survival. Due to the paucity of data and the lack of consensus regarding treatment protocols up to date, clinicians and gynecological oncologist can only refer to published case reports to guide their treatment plans. We therefore report a case and follow-up of a primary malignant melanoma of the vagina in an 89-year-old woman who admitted herself to our tertiary referral hospital at the cantonal hospital of Lucerne, Switzerland, because of postmenopausal bleeding.
Case Report
An 89-year-old multiparous woman came to our general gynecological outpatient clinic on account of a postmenopausal bleeding. The symptoms had occurred approximately two months ago. No further gynecological symptoms were reported. Her gynecological history included four spontaneous labors and reaching menopause at the age of 55. Her medical history included a cholecystectomy many years ago and a recent sigma diverticulitis treated conservatively six months ago. On her mother’s side an aunt had been diagnosed with breast cancer. Otherwise, her family history was uneventful.
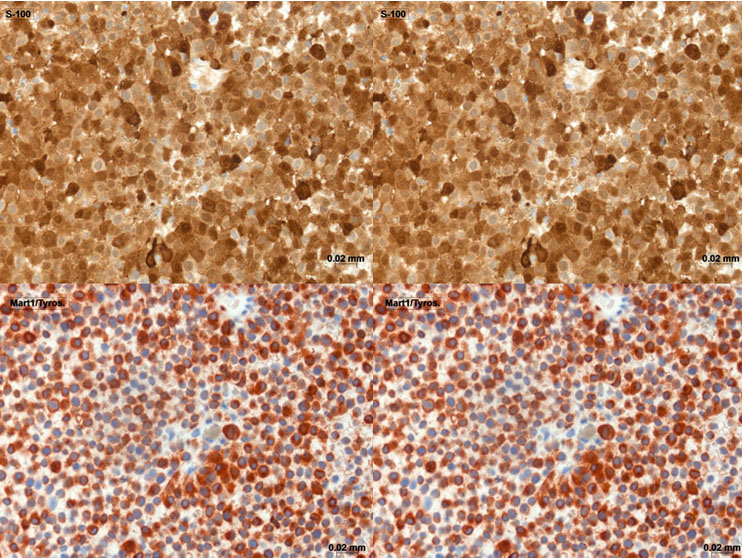
On gynecological examination the ventral vulva showed pigmented areas around the urethra and the labia minora with an extension toward and formation of a bulge in the dorsal vaginal wall. No further abnormalities were detected. A biopsy was taken to determine the entity of the vaginal tumor. A tumorous mass of approximately 5 cm in length and 3 cm in width was palpable in the middle third of the vagina extending as far as the upper third of the vagina. The tumor reached the outer layer of the rectal wall without affecting the mucosa. There were no palpably enlarged lymph nodes. On transvaginal ultrasound the tumor measured 6.6 × 3.2 cm (Figure 1). Abdominal ultrasound of the inguinal lymph nodes showed normal size and shape with no aspects of metastasis. On MRI scan the tumor size was confirmed in absence of distant metastasis (Figure 1). The department of pathology confirmed the diagnosis of a melanoma of the vagina in the biopsy taken. The additional positive immunostaging supported the diagnosis of a PMMV. Tumor cells expressed S-100 and MART 1/Tyros (Figure 2); CD 45 and MNF-116 were negative. Regarding the operative procedure, in order to achieve clear margins, a total exenteration would have been needed to be performed, due to the localization of the tumor close to the rectal wall and additional areas suspicious of melanoma in situ on the ventral vulva including the urethra. We discussed all the surgical options including the previously mentioned exenteration which would have resulted in a final colostomy and an ileum conduit, respectively. Due to her advanced age the patient denied complete oncological excision along with the associated implications.
Thus we performed external beam radiotherapy (50 Gy in 20 fractions) for six weeks, which was well tolerated. The MRI scan performed six weeks after completing radiotherapy showed a partial response of the PMMV with a residual tumor dimensions of 1.8 × 1.8 cm but revealed newly developed metastasis in the femoral heads on both sides. Ten days later she presented with back pain, abdominal pain, nausea, and vomiting. An additional computed tomography (CT) scan of thorax and abdomen was performed showing new pulmonary and hepatic metastasis (Figure 3). The patient refused further therapy and was treated in the palliative care unit at our hospital. She died one week later due to hepatic insufficiency.
Discussion
Primary malignant melanoma of the vagina is a rare and very aggressive tumor. Melanomas can arise from any part of the urogenital tract. Of all mucosal melanomas approximately 18% are located in the female genital tract [3]. Vaginal melanomas account for <3% of all vaginal tumors and for 0.4–0.8% of all melanomas in women [4]. The first case of vaginal melanoma was described in 1887 and approximately 500 cases are reported in recent literature [1]. Vaginal melanoma appears in postmenopausal women, typically in the sixth and seventh decades and is mostly located in the lower third of the anterior vaginal wall [5]. Symptoms include vaginal bleeding, discharge, and a palpable mass in the vagina [6]. Multifocality is encountered in 20%, ulceration in more than 50%, respectively [2]. The appearance of the tumor is mostly pigmented and only a minority of 10–23% of the PMMV are amelanotic [7]. Melanomas have to be differentiated from other pigmented lesions including melanosis, lentigo, and nevi. Among the immunohistochemical markers S100 and SRY (sex-determining region Y)-box 10 (SOX10)) are the most sensitive markers for the diagnosis of melanotic lesions although the specificity is poor [8].
Melanoma antibody recognized by T-cells (MART-1), microphthalmia associated transcription factor (MITF), or human melanoma black-45 (HMB-45) is often used and provide better specificity combined with an acceptable sensitivity for differentiating most conventional melanomas [2]. There was no correlation between the depth of tumor invasion and patient survival. The tumor size is the most important prognostic factor: in a review including 67 women with vaginal melanoma median OS was 41 months for tumors [6],[9]. In case of lymphogenic metastasis median OS is significantly lower compared to women without lymph node involvement (7.8 vs 30 months) [10]. So far no standardized treatment protocol has been defined or established. A complete surgical resection to achieve clear margins is the main treatment modality, whenever possible. The surgical options range from wide local excision to radical vaginectomy or pelvic exenteration. Since radical surgical procedures and local excision were similar regarding recurrence rate and survival, the conservative approaches such as wide local excisions (WLE) are to be favored [11]. A wide local excision with an adequate safety margin can be considered as optimal treatment option [2],[9]. Women treated surgically had a significantly longer OS than those treated nonsurgically [12]. Exenteration with or without radiotherapy should be reserved fora highly selected patient collective with melanomas affecting urethra, bladder, and/or rectum [7]. An exenteration was recommended to our patient in order to reach clear margins, however, she refused this treatment option. Lymph node dissection is not recommended in women with PMMV due to low rates of lymph node metastasis. Additionally, it increases morbidity without showing survival benefit. As of lately the sentinel lymph node biopsy is discussed in context of melanomas of the female genitourinary tract. In case of macroscopically involved groin lymph nodes the removal could improve the locoregional control of disease [7]. Adjuvant radiotherapy can provide better local control after surgery. In the experience of MD Anderson Cancer Center, radiotherapy after wide local excision (WLE) reduced local recurrences with an increased median OS (16.1–29.4 months) although the results showed no statistical significance [10]. Radiotherapy is an option in cases where clear margins could not be achieved, or in cases of pelvic metastasis. Preoperatively, it can be applied to reduce tumor volume in cases with a tumor size >3 cm and it can also be selected as a definitive primary treatment option in women who refuse surgical treatment [11],[12]. As mentioned above our patient refused the exenteration and accepted radiotherapy as single treatment modality, instead. In high risk cases without the possibility of surgical treatment systemic therapy can be discussed. Cytotoxic agents including dacarbazine, temozolomide, and platinum compounds or taxanes have been used as single agents or in combination with limited success rates. The response rate varied from 11% to 22% and the median OS was between 5.6 and 11 months [13]. Huang et al. [14] analyzed the significance of immunotherapy in their retrospective study. The immunotherapy included IL-2, bacillus Calmette-Guerin (BCG) dendritic cells, or lymphokine-activated killer cells and was administered to 19 of 31 women. Five-year OS was 47% in women with surgery and immunotherapy versus 29% in women treated with surgery alone (p < 0.001). These results suggest a positive impact of the immunotherapy on the course of disease, making it a possible treatment modality in these well-selected women. The 5-year survival rate for this aggressive and rapidly growing tumor is very poor, ranging from 0% to 25% irrespective of the chosen therapy [15].
Conclusion
The PMMV is a rare and aggressive disease which affects women in their sixth and seventh decades. Tumor size is the only proven prognostic factor and a complete surgical resection achieving clear margins is the treatment of choice whenever possible offering the best 5-year overall survival. Surgery combined with postoperative radiotherapy is the most widely accepted treatment modality. Radiotherapy has also been suggested as primary definitive treatment in patients with surgically unresectable disease and in patients who refuse surgery. A good local response is achievable but the life limiting risk of distant metastasis remains. Further investigation is needed to define the best therapeutic standard of treatment of the PMMV.
REFERENCES
1.
Kalampokas E, Kalampokas T, Damaskos C. Primary vaginal melanoma, a rare and aggressive entity. A case report and review of the literature. In Vivo 2017;31(1):133–9. [CrossRef]
[Pubmed]

2.
Gadducci A, Carinelli S, Guerrieri ME, Aletti GD. Melanoma of the lower genital tract: Prognostic factors and treatment modalities. Gynecol Oncol 2018;150(1):180–9. [CrossRef]
[Pubmed]

3.
McLaughlin CC, Wu CX, Jemal A, Martin HJ, Roche JM, Chen VW. Incidence of noncutaneous melanomas in the U.S. Cancer 2005;103(5):1000–7. [CrossRef]
[Pubmed]

4.
Göksalan H, Si?mano?lu A, Pekin T, Kaya H, Ceyhan N. Primary malignant melanoma of the vagina: A case report and review of the current treatment options. Eur J Obstet Gynecol Reprod Biol 2005;121(2):243–8. [CrossRef]
[Pubmed]

5.
Takai N, Kai N, Hirata Y, Kashima K, Narahara H. Primary malignant melanoma of the vagina. Eur J Gynaecol Oncol 2008;29(5):558–9.
[Pubmed]

6.
Reid GC, Schmidt RW, Roberts JA, Hopkins MP, Barrett RJ, Morley GW. Primary melanoma of the vagina: A clinicopathologic analysis. Obstet Gynecol 1989;74(2):190–9.
[Pubmed]

7.
Miner TJ, Delgado R, Zeisler J, et al. Primary vaginal melanoma: A critical analysis of therapy. Ann Surg Oncol 2004;11(1):34–9. [CrossRef]
[Pubmed]

8.
Compton LA, Murphy GF, Lian CG. Diagnostic in immunohistochemistry in cutaneous neoplasia: An update. Dermatopathology (Basel) 2015;2(1):15–42. [CrossRef]
[Pubmed]

9.
Buchnan DJ, Schlaerth J, Kurosaki T. Primary vaginal melanoma: Thirteen-year disease-free survival after wide local excision and review of recent literature. Am J Obstet Gynecol 1998;178(6):1177–84. [CrossRef]
[Pubmed]

10.
Frumovitz M, Etchepareborda M, Sun CC, et al. Primary malignant melanoma of the vagina. Obstet Gynecol 2010;116(6):1358–65. [CrossRef]
[Pubmed]

11.
Piura B. Management of primary melanoma of the female urogenital tract. Lancet Oncol 2008;9(10):973–81. [CrossRef]
[Pubmed]

12.
Kirschner AN, Kidd EA, Dewees T, Perkins SM. Treatment approach and outcomes of vaginal melanoma. Int J Gynecol Cancer 2013;23(8):1484–9. [CrossRef]
[Pubmed]

13.
Lin LT, Liu CB, Chen SN, Chiang AJ, Liou WS, Yu KJ. Primary malignant melanoma of the vagina with repeated local recurrences and brain metastasis. J Chin Med Assoc 2011;74(8):376–9. [CrossRef]
[Pubmed]

14.
Huang Q, Huang H, Wan T, Deng T, Liu J. Clinical outcome of 31 patients with primary malignant melanoma of the vagina. J Gynecol Oncol 2013;24(4):330–5. [CrossRef]
[Pubmed]

15.
Stefanovi? A, Jeremi? J, Jeremi? K, Liki? I, Mitrovi? M, Stojni? J. Primary melanoma of the vagina: A case report and review of literature. Eur J Gynaecol Oncol 2015;36(6):755–7.
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Barbara Kipp - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corina Christmann - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2020 Barbara Kipp et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.