|
Case Report
Noninvasive prenatal screening and ultrasonography scan of fetal sex result discordance
1 Medical Student, Texas Tech University Health Sciences Center, School of Medicine, 3601 4th Street, Lubbock, TX, USA
2 Perinatal Medicine, Texas Tech University Health Sciences Center, Department of Obstetrics, 3601 4th Street, 3rd Floor, Lubbock, TX, USA
3 Covenant Maternal-Fetal Diagnostic Center, 4102 24th Street, Suite 101, Lubbock, TX, USA
4 Perinatal Medicine Physician, Covenant Maternal-Fetal Diagnostic Center, Covenant Women’s and Children’s Hospital, 4102 24th Street, Suite 101, Lubbock, TX 79410, USA
Address correspondence to:
Brooke Jensen
Texas Tech University Health Sciences Center, School of Medicine, 3601 4th Street, 3rd Floor, Lubbock, TX 79430,
USA
Message to Corresponding Author
Article ID: 100104Z08BJ2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Jensen B, Atkinson B, Worley K. Noninvasive prenatal screening and ultrasonography scan of fetal sex result discordance. J Case Rep Images Obstet Gynecol 2022;8:100104Z08BJ2022.ABSTRACT
Introduction: Noninvasive prenatal screening (NIPS) for fetal aneuploidies is a screen performed by sequencing placental cell free DNA found in maternal plasma. This is an extremely sensitive and specific screening test with over 99% sensitivity.
Case Report: We present a case of NIPS discordance regarding fetal sex observed on ultrasonography, as well as postnatal phenotype and genetic testing. A preliminary NIPS at 12 weeks gestation revealed no aneuploidies and only X chromosomes; however, 20-week and 23-week ultrasonography demonstrated non-virilized male genitalia. A second NIPS was performed and generated the same findings as the first NIPS. Postnatal exam and fluorescence in situ hybridization (FISH) determined the fetus was of both male phenotype and genotype.
Conclusion: A possible explanation for this patient is mosaic monosomy with a Y-chromosome component (45X/46XY) with normal male phenotype. Sex chromosome mosaicism may not have been detected as placental cfDNA is only a small fraction in maternal circulation.
Keywords: Chromosomal abnormalities, Prenatal diagnosis, Sex chromosomes, Ultrasound
Introduction
Fetal DNA circulating in maternal plasma was first discovered in 1997 by Lo et al. using the Y chromosome as a marker [1]. In that publication, it was explained that when cells undergo apoptosis the intracellular contents are released into the circulatory system. These circulating DNA fragments, originating from intracellular contents, can be found in all human plasma, and are known as cell-free DNA (cfDNA).
In pregnancy, cell-free fetal DNA analyzed from maternal serum originates from apoptotic placental cells rather than apoptotic fetal cells [2]. In 2011, these cells became an integral part of prenatal aneuploidy screening tests, known as cfDNA or “noninvasive prenatal screening” (NIPS). Studies have reported sensitivities of cfDNA analysis for fetal sex chromosomes greater than 99% [3]. When a Y-chromosome component is detected on cfDNA screening, it is considered non-maternal and of placental origin, leading to a fetal sex interpretation of male [4]. We report a case of NIPS discordance regarding fetal sex with ultrasonography scan, as well as postnatal phenotype and genetic testing.
Case Report
The patient was a pregnant gravida I, para 0, 36-year-old woman with endometriosis and hypothyroidism. She had been seen in a reproductive endocrinology clinic since 2016 due to the inability to conceive despite several treatments over the years. The patient conceived spontaneously in 2020 and was followed with two pelvic ultrasonography scans at 7 weeks and 12 weeks. A NIPS Prequel Prenatal Screen was collected at 12.1 weeks gestation with a fetal fraction of 6.9%. Results on the sample were consistent with female sex and no aneuploidy of chromosomes 13, 18, or 21.
On 06/09/2020, the patient presented to the Maternal-Fetal Medicine clinic for her 20-week ultrasound. Ultrasound showed no abnormalities; however, the ultrasound findings demonstrated male genitalia. This finding was discordant with the previous screening results performed at 12.1 weeks gestation. A second NIPS, INNATAL Prenatal Screen was collected at 21.0 weeks gestation with a fetal fraction of 6%. The results were, once again, consistent with normal chromosomes 13, 18, 21, and female sex.
Repeat ultrasound on 06/26/2020 showed similar results to the 20-week ultrasound demonstrating non-virilized male genitalia. The inconsistencies were concerning to the patient and the option of amniocentesis was discussed and offered. After discussing the risks and benefits with the patient and her spouse, an amniocentesis was declined.
At 28 weeks gestation, the patient had a grossly abnormal 1-hour glucose result at 356 mg/dL. The 3-hour glucose tolerance and glycosylated hemoglobin test were not performed. The patient did have a family history of diabetes, but unfortunately did not receive blood glucose screening prior to 28 weeks gestation. This extreme elevation in blood glucose led to increased monitoring, diabetic counseling, and the initiation of insulin for glucose management. Her blood sugar was subsequently well managed by the perinatology team with Novolin N 100 mg/mL subcutaneously of 32 units with breakfast and 12 units before dinner and Novolin R 100 mg/mL injection solution of 18 units before breakfast and 14 units before dinner.
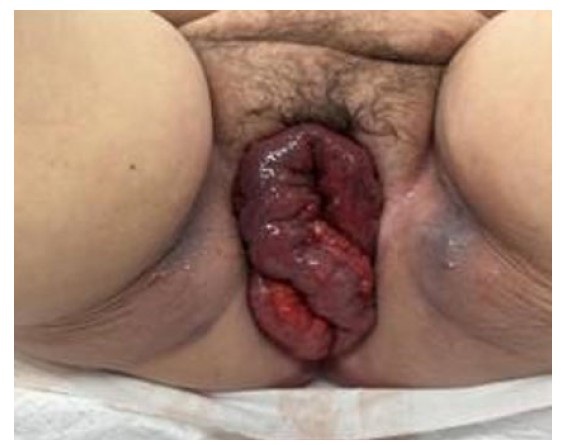
On 09/28/2020 at 35 weeks and 6 days gestation, biophysical profile testing showed a result of 4/8, and the patient was immediately taken to Labor & Delivery. The following day at 36 weeks gestation, the patient vaginally delivered a phenotypically male neonate who weighed 5 pounds and 6 ounces. The neonate was transferred to newborn intensive care unit (NICU) for management of tachypnea which quickly resolved and was thus diagnosed as transient tachypnea of the newborn. Fluorescence in situ hybridization (FISH) was performed using the neonate’s blood while in NICU. Analysis of these results showed no evidence of chromosomal abnormalities in 13, 18, 21, and for the first time, both an X and Y chromosome were observed (Table 1). These results were congruent with the ultrasonography findings and physical exam; the neonate was subsequently determined to be of both male phenotype and genotype.
Discussion
To reduce the likelihood of human error as the cause for an incongruent NIPS, a second screen was performed in laboratories and by different physicians. The most common sources of human error are blood sample mislabeling, laboratory methodologic limitations, and transcription errors [5]. The second NIPS results were concordant with the first screen thus eliminating a previously assumed human laboratory error.
Literature research reveals many biologic reasons for discordance. Some of the most cited reasons for fetal sex discordance are the presence of fetoplacental mosaicism (confined placental mosaicism), vanishing twin syndrome, maternal transplant recipient from a male donor, and disorders of sexual development [3],[4],[6]. Many of these possibilities do not apply to our case. The early ultrasonography excludes the possibility of vanishing twin syndrome, and the mother was not a transplant recipient. Confined placental mosaicism (CPM) refers to the presence of a chromosome abnormality in a portion of placental cells that are not detected in the fetus. The chromosomes seen on both NIPS were normal, therefore reducing the possibility of CPM as the cause of incongruence.
Noninvasive prenatal screening in disorders of sexual development is congruent with the final karyotype (most often due to androgen insensitivity syndrome and incongruence seen on ultrasonography) [7]. Our patient’s NIPS was incongruent with the final karyotype; therefore, disorders of sexual development can also be excluded.
If a definite diagnosis is desired during pregnancy, chorionic villus sampling or amniocenteses is necessary. The option of amniocenteses was discussed with the patient and her spouse but was ultimately denied due to the procedural risks. After seeing normal anatomy on ultrasonography and no aneuploidies found on two separate cfDNA screens, the amniocentesis would be solely used to determine the gender of the fetus. The couple chose to wait until after delivery for the results. Postnatal testing was performed using fluorescence in situ hybridization rather than a full karyotype for cost efficiency and expediency [8],[9].
A limitation worth acknowledging in this case is the lack of full karyotype for the neonate. The karyotype would clarify the neonate’s genetic status thus narrowing the differential diagnosis and enabling appropriate management in the pediatric setting. Our overall recommendation is to pursue with further genetic testing if the patient desires more definite answers.
Conclusion
Sex chromosome mosaicism may not be adequately detected as placental cfDNA is only a small fraction of cfDNA in maternal circulation. Individuals with mosaic monosomy X with a Y-chromosome component genotype can have significant phenotypic variability, ranging from normal external female genitalia with systemic features of Turner syndrome to normal phenotypic male. A possible explanation for this fetal sex discrepancy is mosaic monosomy with a Y-chromosome component (45X/46XY) and a normal male phenotype; however, a confirmatory genetic test is required to reach a final diagnosis.
REFERENCES
1.
Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet 1997;350(9076):485–7. [CrossRef]
[Pubmed]

2.
Canick JA, Palomaki GE, Kloza EM, Lambert-Messerlian GM, Haddow JE. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat Diagn 2013;33(7):667–74. [CrossRef]
[Pubmed]

3.
Neufeld-Kaiser WA, Cheng EY, Liu YJ. Positive predictive value of non-invasive prenatal screening for fetal chromosome disorders using cell-free DNA in maternal serum: Independent clinical experience of a tertiary referral center. BMC Med 2015;13:129. [CrossRef]
[Pubmed]

4.
Byers HM, Neufeld-Kaiser W, Chang EY, Tsuchiya K, Oehler ES, Adam MP. Discordant sex between fetal screening and postnatal phenotype requires evaluation. J Perinatol 2019;39(1):28–33. [CrossRef]
[Pubmed]

5.
Dhamankar R, DiNonno W, Martin KA, Demko ZP, Gomez-Lobo V. Fetal sex results of noninvasive prenatal testing and differences with ultrasonography. Obstet Gynecol 2020;135(5):1198–206. [CrossRef]
[Pubmed]

6.
Richardson EJ, Scott FP, McLennan AC. Sex discordance identification following non-invasive prenatal testing. Prenat Diagn 2017;37(13):1298–304. [CrossRef]
[Pubmed]

7.
Finney EL, Finlayson C, Rosoklija I, et al. Prenatal detection and evaluation of differences of sex development. J Pediatr Urol 2020;16(1):89–96. [CrossRef]
[Pubmed]

8.
Iruretagoyena JI, Grady M, Shah D. Discrepancy in fetal sex assignment between cell free fetal DNA and ultrasound. J Perinatol 2015;35(3):229–30. [CrossRef]
[Pubmed]

9.
Dewald G, Stallard R, Al Saadi A, et al. A multicenter investigation with interphase fluorescence in situ hybridization using X- and Y-chromosome probes. Am J Med Genet 1998;76(4):318–26.
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Brooke Jensen - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Bill Atkinson - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Kevin Worley - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Brooke Jensen et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.