|
Case Report
Twin pregnancy combining complete hydatidiform mole and healthy fetus: Case report and review of the literature
1 Maternity, University Hospital Abderrahim Harouchi, Casablanca, Morocco; Faculty of Medicine and Pharmacy, University Hassan II, Ain Chock BP 5366, 20000 Casablanca, Morocco
Address correspondence to:
Chadia Khalloufi
3, Saria Bnou Zounaim Road, Palmier, 20340 Casablanca,
Morocco
Message to Corresponding Author
Article ID: 100135Z08IJ2023
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Joudar I, Khalloufi C, El Abbassi I, Jalal M, Lamrissi A, Bouhya S. Twin pregnancy combining complete hydatidiform mole and healthy fetus: Case report and review of the literature. J Case Rep Images Obstet Gynecol 2023;9(1):7–10.ABSTRACT
Introduction: Twin pregnancy combining a complete mole and a normal fetal pregnancy with its own healthy trophoblast is a rare entity.
Case Report: We report the case of a 43-year-old female patient admitted for abnormal uterine bleeding during the 20th week of pregnancy. Pelvic ultrasound showed the combination of a complete hydatidiform mole and a normal fetal pregnancy. The decision to medically terminate the pregnancy was taken after consultation with the family. Examination of the placenta and histological study confirmed the diagnosis of complete hydatidiform mole associated with a normal fetus. The evolution was uneventful.
Conclusion: Twin pregnancy combining a complete mole and a normal fetal pregnancy with its own healthy trophoblast is a rare entity that should not be misdiagnosed. There is still no consensus in terms of therapeutic attitude, the dilemma remains and the decision should always include the couple after a thorough explanation of all the risks.
Keywords: Case report, Complete hydatidiform mole, Gestational trophoblastic tumor, Pelvic ultrasound, Twin pregnancy
Introduction
Complete hydatidiform mole coexisting with a live twin fetus (CHMF) is a rare occurrence occurring in 1 in 22,000 to 1 in 100,000 pregnancies according to authors [1],[2],[3],[4]. Given the risks of immediate and long-term maternal complications, it should not be misdiagnosed. Moreover, this presents both the physician and the couple with a significant dilemma between immediate intervention and expectant management. We report the case of a patient hospitalized in the maternity ward of the university hospital Abderrahim Harouchi in Casablanca, presenting a complete hydatidiform mole coexisting with a live twin fetus that resulted in the termination of pregnancy.
Case Report
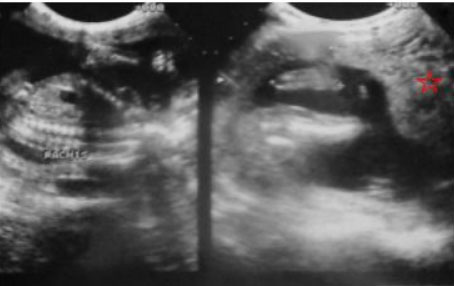
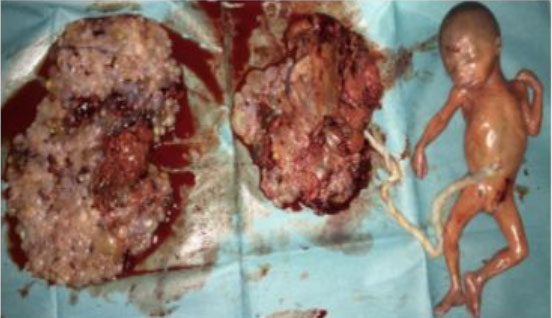
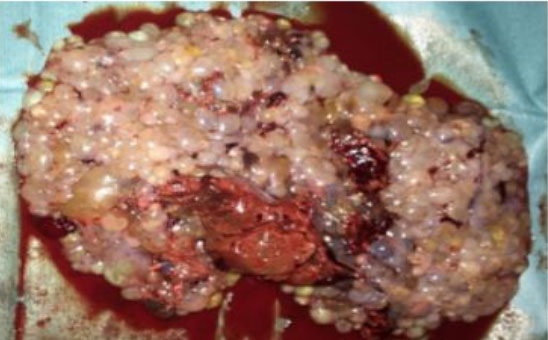
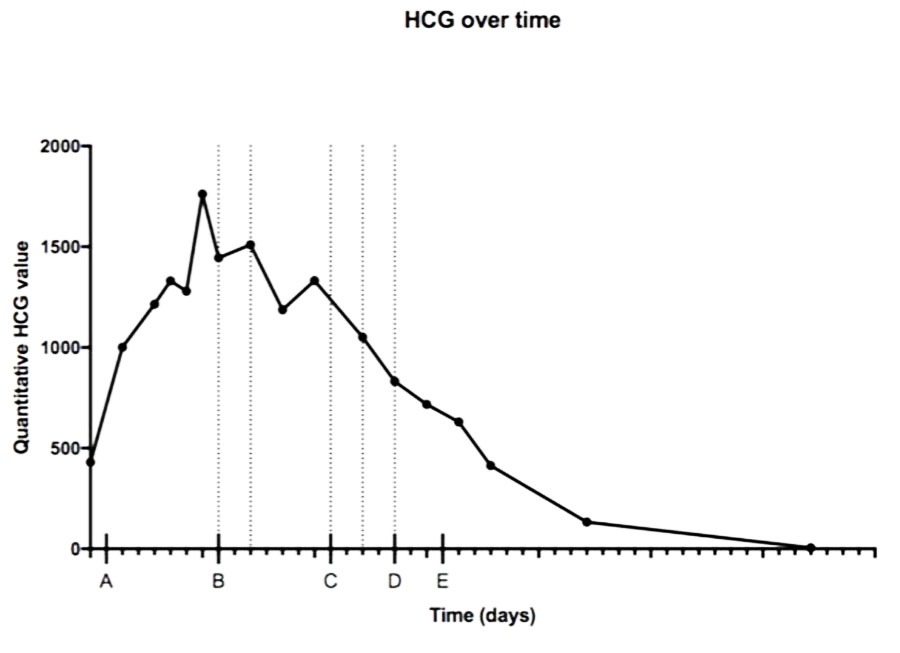
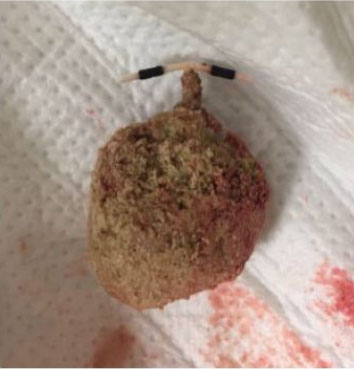
We report the case of a 43-year-old female patient, VG IIIP (3 previous normal deliveries and one miscarriage), having no underlying medical condition, no drug history, and no consanguinity, was admitted for second trimester abnormal uterine bleeding after 20 weeks of amenorrhea, brought in an ambulance from home. This was her second examination and sonography during this pregnancy, the first being in the first trimester (10 weeks of amenorrhea) where the physician, a general doctor missed the diagnosis. Clinical examination found a conscious, hemodynamically stable patient, the cervical canal was slightly open with uterine bleeding and a fundal height greater than gestational age. The ultrasound revealed a bichorial twin pregnancy: two gestational sacs, one with a living fetus of 21SA, another containing an amniotic cavity with a multi-cystic portion suggestive of molar tissue measuring 14 cm (Figure 1). Laboratory test carried showed a level of chorionic gonadotropin hormone (beta-hCG) at 1788,900 mIU/L, without anemia (hemoglobin at 11.5 g/dL), without hyperthyroidism (TSH at 1.18 mIU/L) or diabetes (fasting blood sugar at 0.72 g/dL). Medical termination of pregnancy was indicated: induction of labor with 400 mg of misoprostol intravaginally with no change in cervix dilatation for 6 hours, followed by endo-uterine aspiration. This decision was taken after consultation with the patient and her family, and explaining to them the possible maternal and fetal complications and the poor prognosis of this combination, in particular the risk of progression into a gestational trophoblastic tumor. The expulsion product was in the form of a living fetus of 21SA with its own trophoblast associated with a 14 cm vesicular mass (Figure 2 and Figure 3). The histological examination found a complete hydatidiform mole. Monitoring of the plasma beta-hCG curve revealed a regression of the level after expulsion with absence of myometrial invasion on the follow-up ultrasound, as well as a negativation of beta-hCG, after one month.
 )
)Discussion
Complete hydatidiform mole coexisting with a live twin fetus is a rare entity, with a difficult management given the complications such as fetal death, bleeding, pre-eclampsia, hyperthyroidism, and the risk of progression to a gestational trophoblastic tumor [5]. Diagnosis is based on the clinical features, serum human chorionic gonadotropin (HCG) level, and ultrasound; and will be confirmed after anatomopathological study. The accurate diagnosis isn’t usually made before the end of the first trimester or the beginning of the second trimester, and generally CHMF is diagnosed later than a molar pregnancy in 71% of cases [2],[3]. Clinically, the signs are not very specific and correspond to those of a simple complete hydatidiform mole (CHM) [6]. The ultrasound shows two distinct placental masses, one of normal appearance associated with a healthy fetus, the other of multi-cystic molar appearance with no embryonic element [7]. We can also find functional ovarian cysts, often bilateral, in 25% of cases [8]. In our case, the diagnosis was delayed. The main differential diagnosis of a twin pregnancy with CHM and a healthy fetus is the partial hydatidiform mole (MHP), which must be distinguished from it because the management is completely different [4]. In our case, the diagnosis of CHM was essentially based on the laboratory results and the ultrasound. The management raises an ethical issue: is it possible to continue the pregnancy despite the risk of obstetric or oncological complications?
In 38% of cases, fetal viability is possible. The median gestational age reached at delivery is 35 weeks (25–41 weeks) [9]. This therapeutic management is not without complications, the maternal and fetal prognosis are usually at risk, with 21% of metrorrhagia, a major risk of postpartum hemorrhages, 6% of pre-eclampsia, 43% of late spontaneous abortions, 13% death in utero and prematurity [3],[9]. As well as gestational trophoblastic tumor occurring in 50–57% cases [10]. Given the poor maternal prognosis of CHMF, and when the diagnosis is made early, in the first or second trimester, termination of pregnancy is generally considered the most acceptable course of action [6], as was the case for our patient, whose pregnancy was terminated at 20 weeks. The decision to maintain or terminate the pregnancy isn’t an easy decision to make, and has to be guided by the gestational age, the symptoms, the presence or absence of fetal growth restriction or a malformation, as well as the couple’s wishes after thorough information. The patient should undergo a careful clinical, biological, and ultrasound monitoring if the pregnancy is maintained. If the pregnancy is terminated, monitoring is essentially based on the dosage of HCG once a week until negativation [6], then once a month for a year with effective contraception [7]. Persistence of elevated HCG levels correlates with a higher risk of gestational trophoblastic tumor [1],[8]. In our case, with a follow-up of 16 months, the evolution was favorable, marked by a regression of the HCG levels without a development of a gestational trophoblastic tumor.
Conclusion
Complete hydatidiform mole coexisting with a live twin fetus remains a rare pathology, with a high risk of complications for the mother and the fetus. Diagnosis is possible since the first trimester, that’s why the first trimester examination and sonography should be a very meticulous exam performed by an experimented physician. With the development of ultrasound and Doppler, more and more teams lean toward the continuation of pregnancy, but the dilemma remains and the decision should always include the couple and a thorough explanation of all the risks.
REFERENCES
1.
Malhotra N, Deka D, Takkar D, Kochar S, Goel S, Sharma MC. Hydatiform mole with coexisting live fetus in dichorionic twin gestation. Eur J Obstet Gynecol Reprod Biol 2001;94(2):301–3. [CrossRef]
[Pubmed]

2.
Chesnais AL, Le Breton F, Devouassoux-Shisheboran M, et al. Grossesse gémellaire avec môle complète et foetus vivant: À propos d’un cas non diagnostiqué en anténatal. Annales de Pathologie 2011;31(4):299–302. [CrossRef]

3.
Massardier J, Golfier F, Journet D, et al. Twin pregnancy with complete hydatidiform mole and coexistent fetus: Obstetrical and oncological outcomes in a series of 14 cases. Eur J Obstet Gynecol Reprod Biol 2009;143(2):84–7. [CrossRef]
[Pubmed]

4.
de Marcillac F, Akladios CY, Hui-bon-hoa I, Fritz G, Nisand I, Langer B. Twin pregnancy with complete hydatiform mole and coexistent fetus: Report of 4 cases and review of literature. [Article in French]. J Gynecol Obstet Biol Reprod (Paris) 2015;44(9):840–7. [CrossRef]
[Pubmed]

5.
Boubess I, Filali A, Benbrahim F, Ouassour S, Tazi M, Alami MH. Twin pregnancy involving a molar pregnancy and living fetus with progression to invasive mole: About two cases. [Article in French]. Pan Afr Med J 2015;22:24. [CrossRef]
[Pubmed]

6.
7.
Wee L, Jauniaux E. Prenatal diagnosis and management of twin pregnancies complicated by a co-existing molar pregnancy. Prenat Diagn 2005;25(9):772–6. [CrossRef]
[Pubmed]

8.
Jauniaux E. Ultrasound diagnosis and follow-up of gestational trophoblastic disease. Ultrasound Obstet Gynecol 1998;11(5):367–77. [CrossRef]
[Pubmed]

9.
Sebire NJ, Foskett M, Paradinas FJ, et al. Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet 2002;359(9324):2165–6. [CrossRef]
[Pubmed]

10.
Matsui H, Sekiya S, Hando T, Wake N, Tomoda Y. Hydatidiform mole coexistent with a twin live fetus: A national collaborative study in Japan. Hum Reprod 2000;15(3):608–11. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Imane Joudar - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Chadia Khalloufi - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Imane El Abbassi - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mohammed Jalal - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Amine Lamrissi - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Said Bouhya - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2023 Imane Joudar et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.