|
Case Report
Primary amenorrhea due to sclerotic or “non-functional” endometrium: Case presentation and literature review
1 Specialist Obstetrician & Gynaecologist, Department of Obstetrics and Gynaecology, Royal Darwin Hospital, Australia
2 Registrar in Obstetrics & Gynaecology, Department of Obstetrics and Gynaecology, Royal Darwin Hospital, Australia
Address correspondence to:
Nader Gad
PO BOX 41326, Casuarina, NT 0811,
Australia
Message to Corresponding Author
Article ID: 100064Z08NG2020
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Gad N, Yam C, Thomas S. Primary amenorrhea due to sclerotic or “non-functional” endometrium: Case presentation and literature review. J Case Rep Images Obstet Gynecol 2020;6: 100064Z08NG2020.ABSTRACT
Primary amenorrhea is less common than secondary amenorrhea. It can be caused by various anatomical, genetic, or hormonal factors during the development of the reproductive organs. Congenital absence of the endometrium or sclerotic endometrium is a very rare cause of primary amenorrhea with only three cases previously reported in English literature. We describe a case of sclerotic or non-functional endometrium in the context of Mullerian abnormality and normal female karyotype 46 XX.
Keywords: Absent endometrium, Non-functional endometrium, Primary amenorrhea, Sclerotic endometrium
Introduction
Amenorrhea can be primary or secondary. Amenorrhea is considered to be primary in a girl who has never menstruated and secondary when there is absence of menstruation for more than six months. Primary amenorrhea is best described as the absence of menstruation by 16 years of age irrespective of the presence or absence of normal secondary sexual characteristics, or by 14 years of age in the absence of secondary sexual characteristics [1]. The American Society of Reproductive Medicine has the above definition but with the age of the girl one year earlier than above. It recommends evaluation to occur if there has been a failure to menstruate by 15 years of age in the presence of normal secondary sexual characteristics, or within 5 years after breast development if that occurs before age 10 [2].
Primary amenorrhea can be caused by various anatomical, genetic, or hormonal factors during the development of the reproductive organs, with an estimated incidence of 0.1–0.3%, which is much less common than that of secondary amenorrhea at 3–4% [3].
Sclerotic endometrium due to damage of the basal layer is a known cause of secondary amenorrhea. This may be iatrogenic, following overzealous endometrial curettage, or therapeutic, following endometrial ablation for heavy menstrual bleeding [4]. Intrauterine adhesions and synechiae develop, leading to Asherman’s syndrome, which results in amenorrhea, menstrual abnormalities, recurrent miscarriage, or infertility [5].
Absent or sclerotic endometrium is not commonly considered in the evaluation of primary amenorrhea by most gynecologists. There are only three cases previously reported in English literature [6],[7],[8].
We describe a case of sclerotic or non-functional endometrium in the context of Mullerian abnormality and normal female karyotype 46 XX.
Case Report
A 21-year-old Caucasian female presented to the gynecology clinic for investigation of primary amenorrhea and infertility. She described regular monthly premenstrual symptoms including increased white vaginal discharge, breast tenderness, and irritable mood, but had never experienced menstrual bleeding. She denied cyclical pelvic or lower abdominal pain, lower abdominal swelling, or distension. She reported no family history of menstrual or congenital uterine abnormalities.
On examination, she had a normal body habitus and body mass index (BMI), with normal secondary sexual characteristics. At speculum and bimanual examination, the vagina appeared unremarkable, and the cervix was neither visualized nor palpable. The uterus was mobile and normal sized. Blood tests as described below, karyotype and pelvic ultrasound were performed and a progesterone challenge prescribed.
Normal female karyotype of 46 XX was confirmed, and the hormonal profile was normal and confirmed ovulation [follicle-stimulating hormone (FSH) 3.1 IU/L, luteinizing hormone (LH) 3.0 IU/L, progesterone 22 nmol/L, estradiol 387 pmol/L, thyroid stimulating hormone (TSH) 0.88 mU/L, free thyroxine (FT4) 14.2 pmol/L, prolactin 240 mIU/L, testosterone 0.9 nmol/L]. Normal levels of 17-hydroxyprogesterone (3.9 nmol/L) and 21-hydroxylase enzyme (< 0.4 U/mL) were also confirmed.
There was no withdrawal bleeding following the progesterone challenge test. Pelvic ultrasound was challenging, with difficulty in ascertaining uterine contour and cervical morphology. There appeared to be two hemiuteri, with a possible vestigial cervix. A discrete cervical canal was not apparent. The two uterine bodies seemed to be connected by a prominent bridge of tissue in the midline. There appeared to be endometrial tissue superiorly in the uterine cavity at the fundus of the right hemiuterus, and similarly, a small echogenic focus was seen in the fundal region of the left hemiuterus, and may reflect a small amount of endometrial tissue. These areas of presumed endometrial tissue did not extend inferiorly toward the uterine bridge in the midline. Normal ovaries were visualized.
A magnetic resonance imaging (MRI) demonstrated two separate uterine bodies, right larger than left, with a prominent bridge of myometrium in the midline connecting the lower uterine bodies, suggestive of a bicornuate uterus. The study could neither confirm nor exclude the presence of either a single or double cervix.
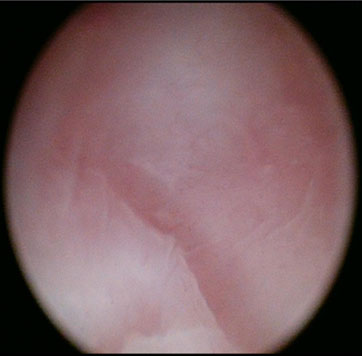
The patient was consented for examination under anesthesia (EUA), hysteroscopy, and dilation and curettage of both hemiuteri if possible. At EUA and vaginoscopy, the vagina was a blind ending pouch of about 5–6 cm in length, and the cervix was absent. The vaginal vault was smooth with no dimpling or firmness to suggest the presence of an underlying cervix (Figure 1). In the absence of communication between the vaginal vault and the uterine body, neither hysteroscopic assessment of either uterine cavity nor endometrial sampling could be undertaken.
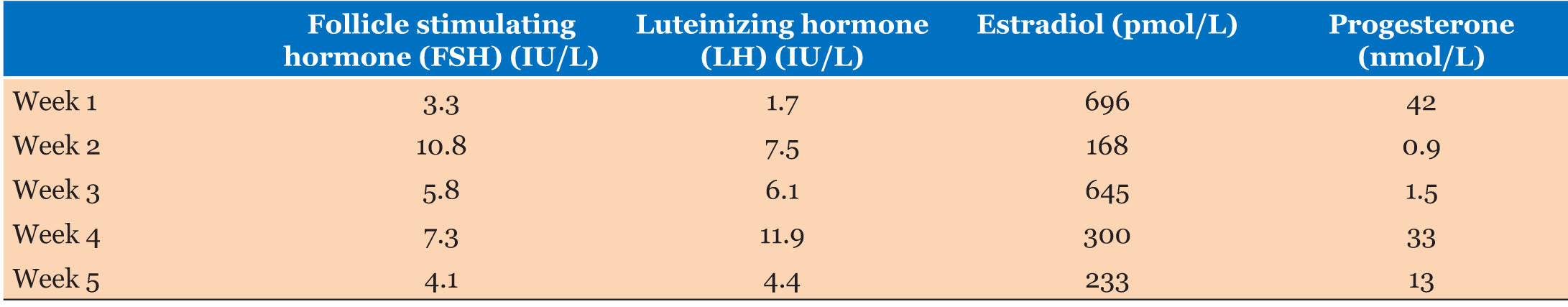
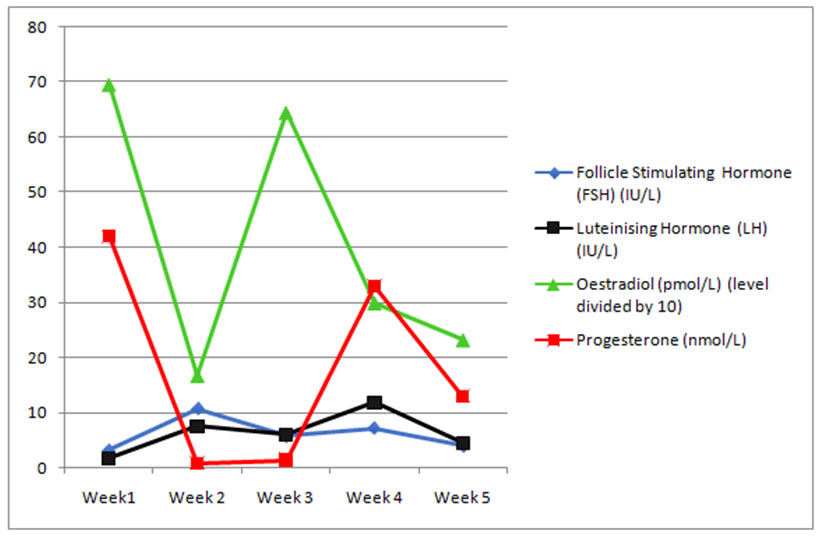
As her foremost concerns were surrounding her reproductive potential and options, hormonal profiles were assayed over a month and correlated with ultrasound assessment of endometrial thickness and morphology. Follicle-stimulating hormone, LH, estrogen, and progesterone levels were measured on five occasions over a 4-week period, and a normal ovulatory pattern confirmed (Table 1 and Figure 2). Transvaginal pelvic ultrasound to assess endometrial thickness and morphology were performed on the same day as the blood tests. The endometrial thickness and morphology did not change over this 4-week period, and did not reflect the hormonal pattern depicted in Figure 2, suggestive of non-functional endometrium.
The Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome (congenital absence or underdevelopment of the uterus and vagina) accounts for 10% of cases of primary amenorrhea. There are reported rare cases of patients exhibiting an isolated case of MRKH, in which there may be atresia or narrowing of the upper portion of the vagina and presence of a rudimentary or underdeveloped uterus. To check for this rare type of MRKH, the single nucleotide polymorphism (SNP) array test was performed and no pathogenic abnormality was detected, making this possibility very unlikely.
Discussion
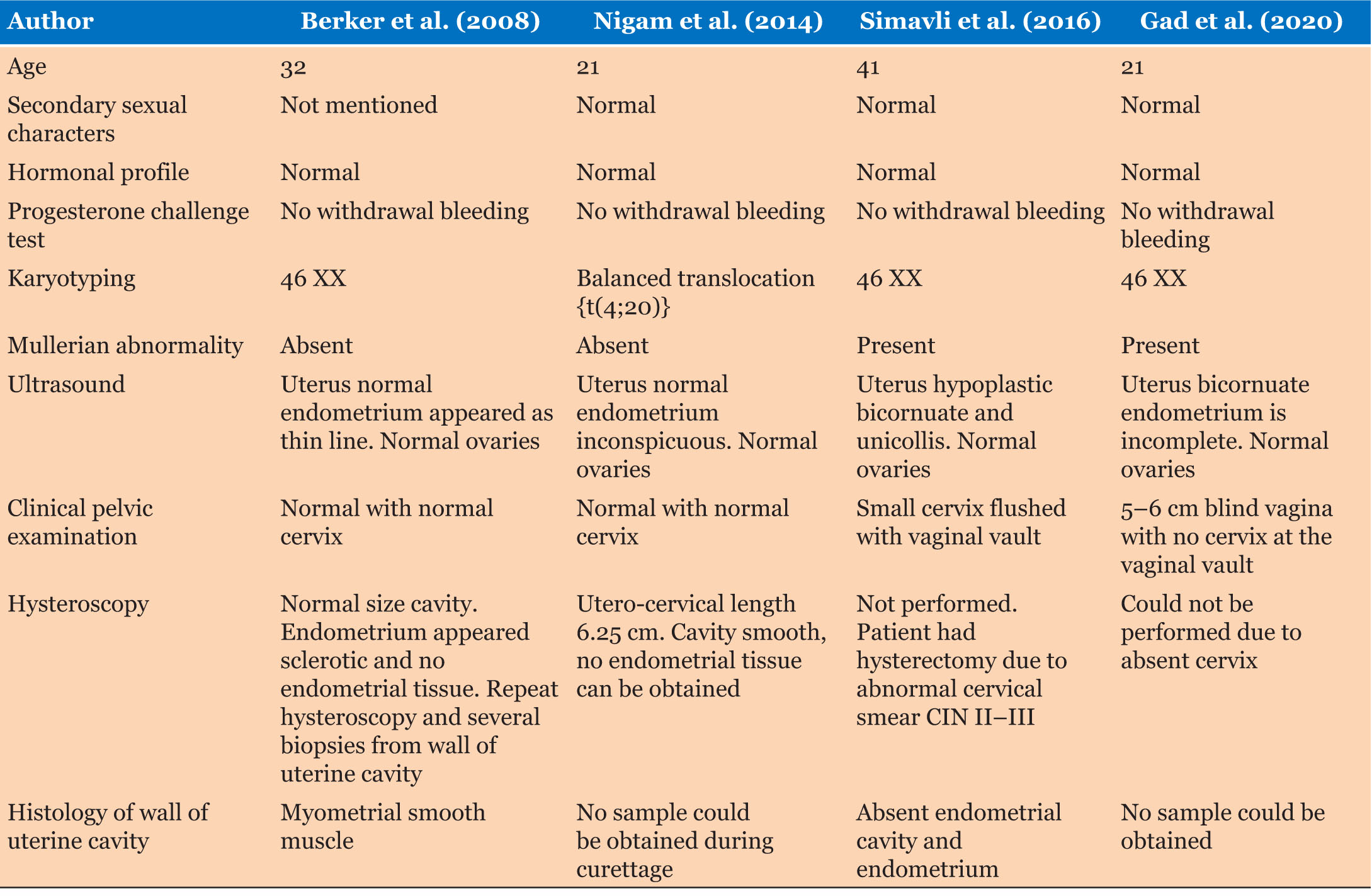
At literature review three case reports describing absent or sclerotic endometrium as a cause of primary amenorrhea were identified. The salient features of these reports are summarized in Table 2.
Berker et al. (2008) described a 32-year-old lady with normal karyotype and reproductive anatomy, but hysteroscopy showing absent endometrium with biopsies demonstrating myometrial smooth muscle [6]. This case differs from ours in that their patient had a normal uterus and cervix. Nigam et al. (2014) documented a 21-year-old with primary amenorrhea, and found to have a balanced translocation of t(4;20)(q12;q13.1) with normal reproductive anatomy and no endometrium on hysteroscopy [7]. This woman had two differences than our patient in that this patient had abnormal chromosomes but normal anatomy of the uterus and cervix. Simavli et al. (2016) described a 41-year-old with Mullerian abnormality, specifically hypoplastic bicornuate uterus. The patient needed abdominal hysterectomy for treatment of CIN 2–3 as her cervix was very small and flush with the vaginal vault. Histology confirmed absent endometrial lumen and endometrium [8]. This patient had similarities to our patient due to presence of Mullerian abnormality of the uterus and normal chromosomes 46 XX.
In our patient, at each pelvic ultrasound examination, there appeared to be a small amount tissue with the appearance of endometrium, in the upper right horn. This endometrium appeared to be incomplete, in other words, it was present in the upper part of the horn, but did not extending to the lower body of the small hemiuterus. The response of this patient’s endometrial tissue was poor and not reflective of the cyclic change in estrogen and progesterone levels. Furthermore, at none of the pelvic ultrasound examinations, was fluid observed inside either of the uterine horns throughout the ovarian cycle. These findings are indicative of non-functional endometrium.
We acknowledge that we could not obtain endometrial samples for histological examination to confirm sclerotic endometrium, and thus prefer to use the term “non-functional” endometrium rather than sclerotic endometrium.
In the presence of normal karyotyping 46 XX, primary amenorrhea due to absence of the uterus is usually associated with absence of the vagina due to failure of the development of the Mullerian duct system as in MRKH syndrome. The incidence of MRKH syndrome is 1:5000 female live births.
In rare cases of isolated MRKH syndrome there may be atresia or narrowing of the upper portion of the vagina and the presence of a rudimentary or underdeveloped uterus [9],[10]. If any of the responsible genes are identified in the affected woman, then it is autosomal dominant and thus the offspring have a 50% chance of having the syndrome. This is an essential information for the young woman to be informed of before considering surrogate pregnancy.
In the case presentation by Simavli et al. they tested for HOXA 10/HOXA 11/HOXA 13 which were negative. We tested for this rare type of MRKH syndrome in our patient, SNP array test was performed and no pathogenic abnormality was detected, making this possibility very unlikely [11].
Conclusion
We describe a rare case of a 21-year-old woman with primary amenorrhea, normal secondary sexual characteristics, and a rare Mullerian abnormality with a blind vagina and bicornuate uterus. Hormonal assays confirmed an ovulatory ovarian cycle, and concurrent pelvic ultrasounds did not reflect the expected changes in endometrial thickness and appearance. Karyotype was normal, and a rare type of MRKH syndrome, excluded by a single nucleotide polymorphism (SNP) array. We conclude that the cause of primary amenorrhea is secondary to non-functional endometrium in this woman with a rare Mullerian abnormality.
REFERENCES
1.
2.
Practice Committee of American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril 2008;90(5):219–25. [CrossRef]
[Pubmed]

4.
Lethaby A, Hickey M, Garry R, Pennix J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev 2009;(4):CD001501. [CrossRef]
[Pubmed]

6.
Berker B, Taşkin S, Taşkin EA. Absence of endometrium as a cause of primary amenorrhea. Fertil Steril 2008;89(3):723.e1–3. [CrossRef]
[Pubmed]

7.
Nigam A, Ahmad A, Batra S. Absent endometrium due to balanced translocation [t(4;20)] presenting as primary amenorrhea. J Hum Reprod Sci 2014;7(1):63–5. [CrossRef]
[Pubmed]

8.
Simavli S, Abreu AP, Kwaan MR, et al. Candidate gene analysis in a case of congenital absence of the endometrium. Fertil Res Pract 2016;2:3. [CrossRef]
[Pubmed]

9.
Reindollar RH, Byrd JR, McDonough PG. Delayed sexual development: A study of 252 patients. Am J Obstet Gynecol 1981;140(4):371–80. [CrossRef]
[Pubmed]

10.
Aittomäki K, Eroila H, Kajanoja P. A population-based study of the incidence of Müllerian aplasia in Finland. Fertil Steril 2001;76(3):624–5. [CrossRef]
[Pubmed]

11.
Takahashi K, Hayano T, Sugimoto R, et al. Exome and copy number variation analyses of Mayer-Rokitansky-Küster-Hauser syndrome. Hum Genome Var 2018;5:27. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Nader Gad - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Chantelle Yam - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sujatha Thomas - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2020 Nader Gad et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.