|
Case Series
Ovarian carcinoid tumor: Comparing two cases with different histological patterns
1 Department of Obstetrics and Gynaecology, United Christian Hospital, Hong Kong
2 Department of Pathology, United Christian Hospital, Hong Kong
Address correspondence to:
Shui-ying Cheng
Department of Obstetrics and Gynaecology, United Christian Hospital,
Hong Kong
Message to Corresponding Author
Article ID: 100068Z08CY2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Yu C-h, Cheng S-y. Ovarian carcinoid tumor: Comparing two cases with different histological patterns. J Case Rep Images Obstet Gynecol 2021;7: 100068Z08CY2021.ABSTRACT
Introduction: Ovarian carcinoid is a well differentiated neuroendocrine tumor, which has similar features as those counterparts in gastrointestinal tract.
Case Series: We present here two cases of carcinoid tumor arising at ovary, which presented as ovarian mass. Histopathology examination and immunohistochemical studies revealed neuroendocrine tumor. This article illustrates two cases of ovarian carcinoid which show different histological pattern and clinical presentation.
Conclusion: The clinician needs to be aware of the clinical symptoms of carcinoid syndrome. Coupled with blood test of 5-HIAA, surgical resection is beneficial for patients. The clinical presentation correlates well with the histological subtypes.
Keywords: Carcinoid, Ovary, Tumor
Introduction
Neuroendocrine tumor is most commonly found in gastrointestinal tract or lung. Among primary ovarian tumors, carcinoid tumor is an unusual entity. Among the cases, approximately two-thirds present as an ovarian mass, the remaining has a possibility of distant spread [1]. It is categorized in monodermal teratoma/somatic type tumors arising from a dermoid cyst in WHO Classification of Tumors of Female Reproductive Organs in 2014 (4th Edition) [2].
Clinically, carcinoid tumors can present as ovarian mass. In many cases, the patient also presents with carcinoid syndrome, including flushing, diarrhea, etc. The clinical presentation is correlated with the histological pattern, which is subdivided into insular, trabecular, stromal, or mucinous types. Insular subtype is the most common histological pattern, featuring closely packed nests of tumor cells. Among various histological subtypes, the insular subtype is associated with carcinoid syndrome, while other subtypes do not. There is no definite histological subtyping parameter to determine the risk of malignancy [3].
The adjacent ovarian tissue often shows mature cystic teratoma. The usual presentation is a unilateral ovarian mass with yellowish-gray appearance. Extra-ovarian neuroendocrine tumor has to be excluded before making a diagnosis of primary ovarian carcinoid.
A thorough search of a local tertiary hospital registry in the past 5 years (2016 to 2020) was performed. Two cases of ovarian carcinoid were found in the hospital registries. The following is the presentation of two cases.
CASE SERIES
Case 1
A 68-year-old lady had one-year history of loose stool diarrhea and flushing. Physical examination was performed by general practitioner and found a large pelvic mass on per-rectal examination. She was thus referred to gynecology clinic. Computed tomography (CT) was booked in 12/2019 and showed an 8.5 × 10.8 × 12.4 cm solid, very vascular and heterogeneously enhancing mass in the center of pelvis (Figure 1). Magnetic resonance imaging (MRI) was performed in 1/2020 and showed a large heterogenous mass 10.9 × 11.2 × 9 cm which was inseparable from upper rectum and sigmoid colon. Oesophago-gastro-duodenoscopy and colonoscopy were done and findings were unremarkable. Ultrasound-guided trucut biopsy of the mass was performed pre-operatively, in which the histology report revealed neuroendocrine tumor, grade I. Pre-operative 24 hour urine 5-hydroxyindoleacetic acid (HIAA) was 1034 umol/day. Total abdominal hysterectomy and bilateral salpingo-oophorectomy was done in February 2020 and found an enlarged left ovary with smooth surface. The ovary measured 10 cm across.
Gross examination
Submitted was a specimen of left ovary with left fallopian tube. The left Fallopian tube measured 8 cm in length, 0.5 cm across. The left ovarian mass was oval in shape and measured 13 × 11 × 6 cm. The outer surface was smooth. Cut surfaces showed yellowish and whitish trabecular firm tissue with focal hemorrhagic necrosis.
Microscopic examination
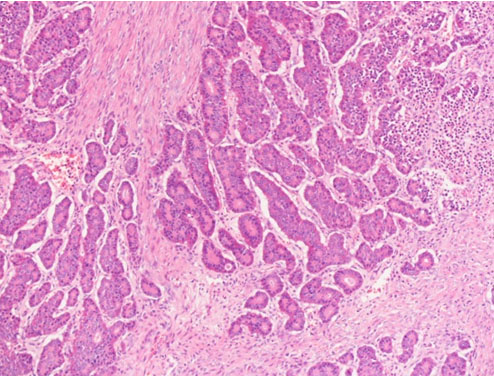
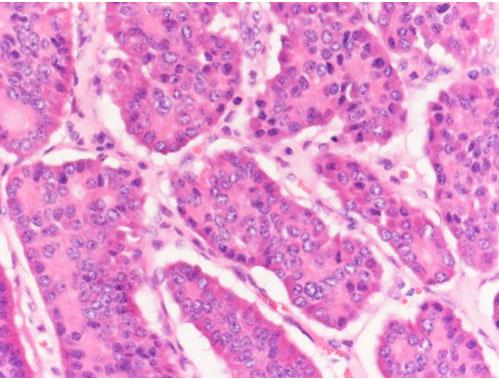
Sections of left ovary showed involvement by tumor tissue. The latter was composed of nests, acini, and trabeculae of tumor cells separated by dense fibrous tissue. The tumor cells featured low columnar to cuboidal cells with regular round nuclei, stippled chromatin, and eosinophilic cytoplasm with abundant red-brown granules. Mitosis was seen at less than 1/10 high power fields. Lymphoplasmacytic infiltrates were noted focally. Ovarian tissue was noted in the peripheral of tumor, and the ovarian capsule was intact (Figure 2 and Figure 3).
There was no teratoma component.
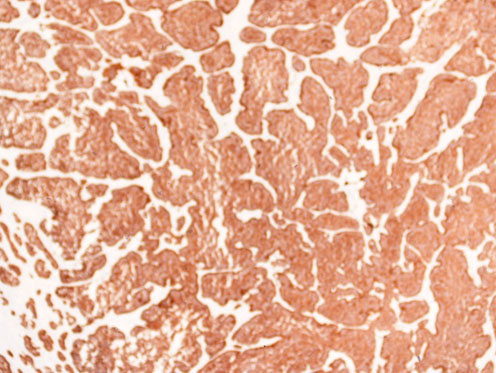
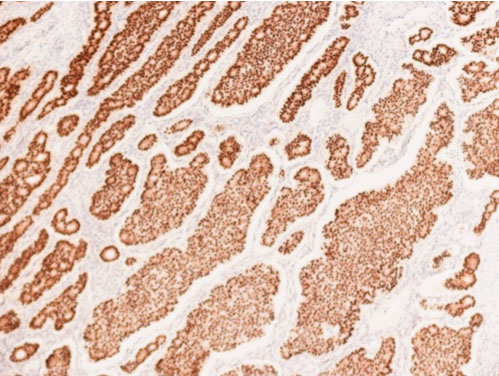
Immunohistochemically, the tumor cells were strongly positively positive for synaptophysin (Figure 4) and CDX-2 (Figure 5). Staining for TTF-1 or thyroglobulin was negative. Ki-67 proliferative index was <1%. The left fallopian tube was unremarkable.
Progress
The case was reviewed by clinical oncologists. Overall clinical presentation was compatible with a tumor with low malignant potential. Therefore, adjuvant therapy was not required. Since it was a functional tumor, they suggested monitoring the disease with 24 hour urine HIAA level.
Patient’s post-operative 24 hour urine HIAA level was 15 umol/day, which was significantly lower than the pre-operative level. Her symptoms of diarrhea and flushing subsided.
Case 2
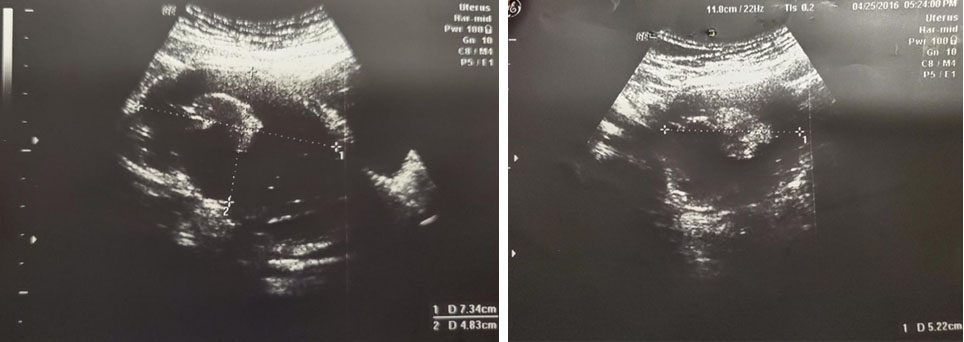
A 37-year-old lady presented with abdominal distension. She was referred to gynecology clinic and a CT was performed. Computed tomography in late 2015 showed a right adnexal cystic mass which measured 6.71 × 5.25 × 5.64 cm. Subsequent ultrasound showed a multiloculated right adnexal cyst with calcification 7.34 × 4.83 × 5.22 cm (Figure 6). Therefore, laparoscopic right ovarian cystectomy was performed in May 2016. Intra-operatively, the surgeons found a right ovarian cyst, which measured 7 × 6 × 5 cm. The cyst had sebaceous materials and hair inside. Pathology showed mature teratoma complicated with squamous cell carcinoma in situ and carcinoid. Her post-operative CA 125 level was 18 U/mL, compared to the pre-operative CA 125 level of 20 U/mL. She was advised to have right salpingo-oophorectomy (RSO) since small residual disease could not be ruled out. Laparoscopic RSO was done in February 2017. Pathology showed no evidence of malignancy. The pre-and post-operative 5-HIAA level was not available.
Gross examination
Specimen was multiple fragmented pieces of cyst wall tissue and tumor tissue. It consisted of greasy material, adipose tissue, and grayish firm irregular tissue. The specimen measured 5.7 × 4 × 3.8 cm in aggregate.
Microscopic examination
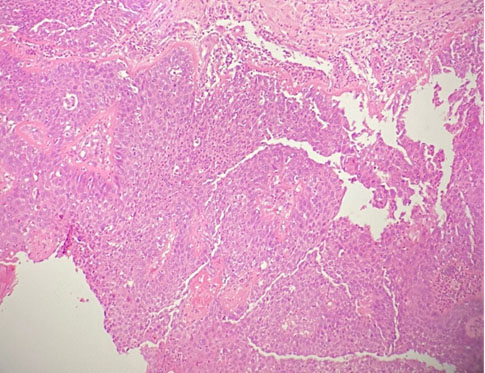
Components of mature epidermis, skin appendages, adipose tissue, cartilaginous tissue, respiratory type epithelium were seen. In areas, aggregates of histiocytes and multinucleated giant cells were found. There was a focus (4 mm) of trabecular pattern of cells with oval nuclei and eosinophilic cytoplasm (Figure 7). These cells were immunoreactive to synaptophysin, in keeping with a carcinoid component.
At some areas, the fibrous cyst wall and focal broad papillary structures were lined by thickened stratified squamous epithelium with loss of maturation, marked nuclear pleomorphism, occasional prominent nucleoli, and readily seen mitotic figures. There was no definite stromal invasion despite examination of multiple levels. No immature neuroepithelium or other immature element was seen. Features were those of squamous cell carcinoma in situ and carcinoid tumor arising from a mature cystic teratoma (Figure 8).
Progress
Upon subsequent follow-ups until year 2020, the patient had no relapse of symptoms. There was no recurrence of tumor.
Discussion
Primary carcinoid tumor of the ovary represents less than 1% of all carcinoid tumors and less than 0.1% of all ovarian neoplasms [4].
An analysis of ovarian carcinoid published in 2000 included 329 cases of primary ovarian carcinoid found 57% occurring on top of teratoma and 47% occurring as pure form [5]. Mature teratoma is common and accounted for ~20% of all ovarian neoplasms. Malignant transformation is rare but possible. The average frequency is 1–2%. The commonest overlying malignancy was squamous cell carcinoma, followed by adenocarcinoma then carcinoid tumor [6].
Malignant tumor within ovarian mature teratoma is more commonly occurred in menopausal woman (p < 0.005). Some studies also suggested that the average tumor size of malignancy associated with ovarian mature teratoma is larger than benign ovarian mature teratoma (p > 0.005) [1],[7]. Though in our second case, the size of the tumor was just average size and the patient is pre-menopausal.
There are no specific ultrasound imaging findings which make mature cystic teratoma distinguishable from carcinoid tumor. Low signal intensity on T2-weighted MRI images which is rarely observed in other malignant ovarian tumors may be a characteristic imaging finding in ovarian carcinoid [8].
Carcinoid tumors secrete a variety of neurohumoral substances including serotonin, histamine, tachykinin, bradykinin, kallikrein, corticotrophin, substance P, motilin, and prostaglandins [9]. Persistent exposure to these substances results in carcinoid syndrome with the classic triad of flushing of arms and face, wheezes, and diarrhea. Functioning ovarian carcinoid can cause these symptoms because the substances secreted by the tumor has bypassed the portal venous system.
5-Hydroxyindoleacetic acid is the main metabolite of serotonin. In chemical analysis of urine 5-HIAA is used to determine serotonin levels in body and therefore is suggested to use as post-operative monitoring method in our first case. Carcinoid syndrome occurs only in insular type carcinoid tumor and in only about 30% of cases [10]. In patients with an associated carcinoid syndrome, octreotide appears to be effective as a hormonal palliation [9],[11].
Clinically, the clinician should be aware of the unusual symptoms that the patient presents as carcinoid syndrome, including diarrhea or flushing, which are sometimes non-specific and easily missed. Awareness of the clinical features can render prompt biochemical investigation for the patient pre-operatively, by which the subsequent treatment plan can be modified.
Conclusion
Here two cases of ovarian carcinoid tumor are reported. Primary ovarian carcinoid tumor is an uncommon tumor. The usual course is indolent with good prognosis. Clinical presentation of carcinoid syndrome is limited in certain histological pattern. Therefore including carcinoid tumor in the differential diagnosis is important, coupled with blood test for 5-HIAA. The treatment of choice is by surgical resection.
REFERENCES
1.
Trabzonlu L, Durmaz G, Vural C, Muezzinoglu B, Corakci A. Malignant tumors associated with ovarian mature teratoma: A single institution experience. Pathol Res Pract 2017;213(5):518–21. [CrossRef]
[Pubmed]

2.
3.
Lenicek T, Tomas D, Soljacić-Vranes H, et al. Strumal carcinoid of the ovary: Report of two cases. Acta Clin Croat 2012;51(4):649–53.
[Pubmed]

4.
Metwally IH, Elalfy AF, Awny S, Elzahaby IA, Abdelghani RM. Primary ovarian carcinoid: A care report of two cases and a decade registry. J Egypt Natl Canc Inst 2016;28(4):267–75. [CrossRef]
[Pubmed]

5.
Soga J, Osaka M, Yakuwa Y. Carcinoids of the ovary: An analysis of 329 reported cases. J Exp Clin Cancer Res 2000;19(3):271–80.
[Pubmed]

6.
Ueda G, Fujita M, Ogawa H, Sawada M, Inoue M, Tanizawa O. Adenocarinoma in a benign cystic teratoma of the ovary: Report of a case with a long survival period. Gynecol Oncol 1993;48(2):259–63. [CrossRef]
[Pubmed]

7.
Sporrong B, Falkmer S, Robboy SJ, et al. Neurohormonal peptides in ovarian carcinoids: An immunohistochemical study of 81 primary carcinoids and of intraovarian metastases from six mid-gut carcinoids. Cancer 1982;49(1):68–74. [CrossRef]
[Pubmed]

8.
Srisajjakul S, Prapaisilp P, Bangchokdee S. Imaging features of unusual lesions and complications associated with ovarian mature cystic teratoma. Clin Imaging 2019;57:115–23. [CrossRef]
[Pubmed]

9.
Kvols LK. Metastatic carcinoid tumors and the malignant carcinoid syndrome. Ann N Y Acad Sci 1994;733:464–70. [CrossRef]
[Pubmed]

10.
Desouki MM, Fadare O, Chamberlain BK, Shakir N, Kanbour-Shakir A. Malignancy associated with ovarian teratomas: Frequency, histotypes, and diagnostic accuracy of intraoperative consultation. Ann Diagn Pathol 2015;19(3):103–6.
[Pubmed]

11.
Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97(4):934–59. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Chun-hung Yu - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Shui-ying Cheng - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Chun-hung Yu et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.