|
Case Report
MRI of complete hydatidiform mole with normal hCG and viable intrauterine pregnancy
1 Medical student, Technion - Israel Institute of Technology School of Medicine, Haifa, Israel
2 Department of Obstetrics and Gynecology, Rambam Health Care Campus, Haifa, Israel
3 Adjunct Professor of Radiology, The George Washington University School of Medicine, 2300 I St NW, Washington, DC 20052, USA; Chairman of Medical Imaging, Rambam Health Care Campus, HaAliya HaShniya St 8, Haifa 3109601, Israel
Address correspondence to:
Marcia C Javitt
MD, Adjunct Professor of Radiology, The George Washington University School of Medicine, 2300 I St NW, Washington, DC 20052, USA; Chairman of Medical Imaging, Rambam Health Care Campus, HaAliya HaShniya St 8, Haifa 3109601,
Israel
Message to Corresponding Author
Article ID: 100089Z08SW2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Weinstein S, Ginsberg Y, Reiss A, Javitt MC. MRI of complete hydatidiform mole with normal hCG and viable intrauterine pregnancy. J Case Rep Images Obstet Gynecol 2021;7:100089Z08SW2021.ABSTRACT
Introduction: Coexistent complete mole with a concomitant live fetus is a rare occurrence. Initially it may be identified by Ultrasound (US). Magnetic resonance imaging (MRI) is a useful alternative to US because MRI depicts the molar mass as separate from the live fetus, its monochorionic monoamniotic gestational sac and normal appearing placenta.
Case Report: A rare case of complete hydatidiform mole with concomitant live intrauterine pregnancy and normal level of serum human chorionic gonadotropin (hCG) is reported. Obstetrical US showed an indeterminate multi-cystic lenticular shaped uterine mass without ovarian theca lutein cysts. Magnetic resonance imaging showed the molar mass as separate from the live fetus within its gestational sac and a normal placenta. Although partial mole was favored because of the normal hormone level, pathology showed a complete mole after delivery of a live fetus.
Conclusion: This case highlights the utility of MRI for diagnosis and optimized management if US and clinical findings are indeterminate.
Keywords: Complete hydatidiform mole, Human serum chorionic gonadotropin, Intrauterine pregnancy
Introduction
Coexistent complete mole with a concomitant live fetus is a rare occurrence, with a rate of about 1 in 22,000–100,000 pregnancies [1]. Typically first identified as an incidental finding on Ultrasound (US), a coexistent complete mole and live pregnancy is a dizygotic twin pregnancy wherein the normal fetus will have a normal placenta [2]. Complete moles contain 46 paternal chromosomes and do not have any fetal parts, whereas partial moles (the main differential diagnostic consideration) contain a triploid set of chromosomes resulting from two sperms that fertilize a haploid oocyte [3].
One of the methods to differentiate between complete and partial moles is the serum human chorionic gonadotropin (hCG) levels. In normal singleton pregnancies without moles, serum hCG levels typically peak in the 9th and 10th weeks of gestation and decline steadily thereafter [4]. In contrast, complete or partial hydatidiform moles are usually diagnosed when an abnormally high serum hCG is detected.
US, the initial study of choice for hydatidiform mole, typically shows a multi-cystic mass without fetal parts and without central color Doppler flow in the cystic component [5]. Doppler typically demonstrates high velocity and low impedance flow in the solid parts of the molar mass. However, the US detection rate of a coexistent mole with a concomitant live fetus is only about 68% [6]. Magnetic resonance imaging (MRI) is a useful alternative to US because MRI depicts the molar mass as separate from the live fetus with its gestational sac and normal placenta. Moreover, the mole’s depth of myometrial invasion on T2-weighted MRI as indicated by heterogeneous signal at an indistinct endo-myometrial boundary is important for planning separation of the molar mass from the uterus [2]. Magnetic resonance imaging also can be useful when US is equivocal or technically limited, such as for obese patients.
In this case, a multicystic complete molar pregnancy was not associated with a markedly increased hCG, a live twin had a separate sac and placenta, and the obstetrical US was equivocal. This case highlights the utility of MRI for diagnosis and optimized conservative management of a complete mole and concomitant live twin.
Case Report
A 35-year-old woman, P5 G4 SAB1, presented with vaginal bleeding at 10 weeks gestational age estimated by date of last menstrual period. No clinical evidence of a twin gestation was found (i.e., no abnormal elevation in hCG, hyperemesis, abnormally large uterus). Transvaginal US showed a live fetus and findings compatible with a 1.0 1.4 cm fundal subchorionic hemorrhage. There was no sonographic evidence of a twin fetus, fetal parts, or empty twin sac.
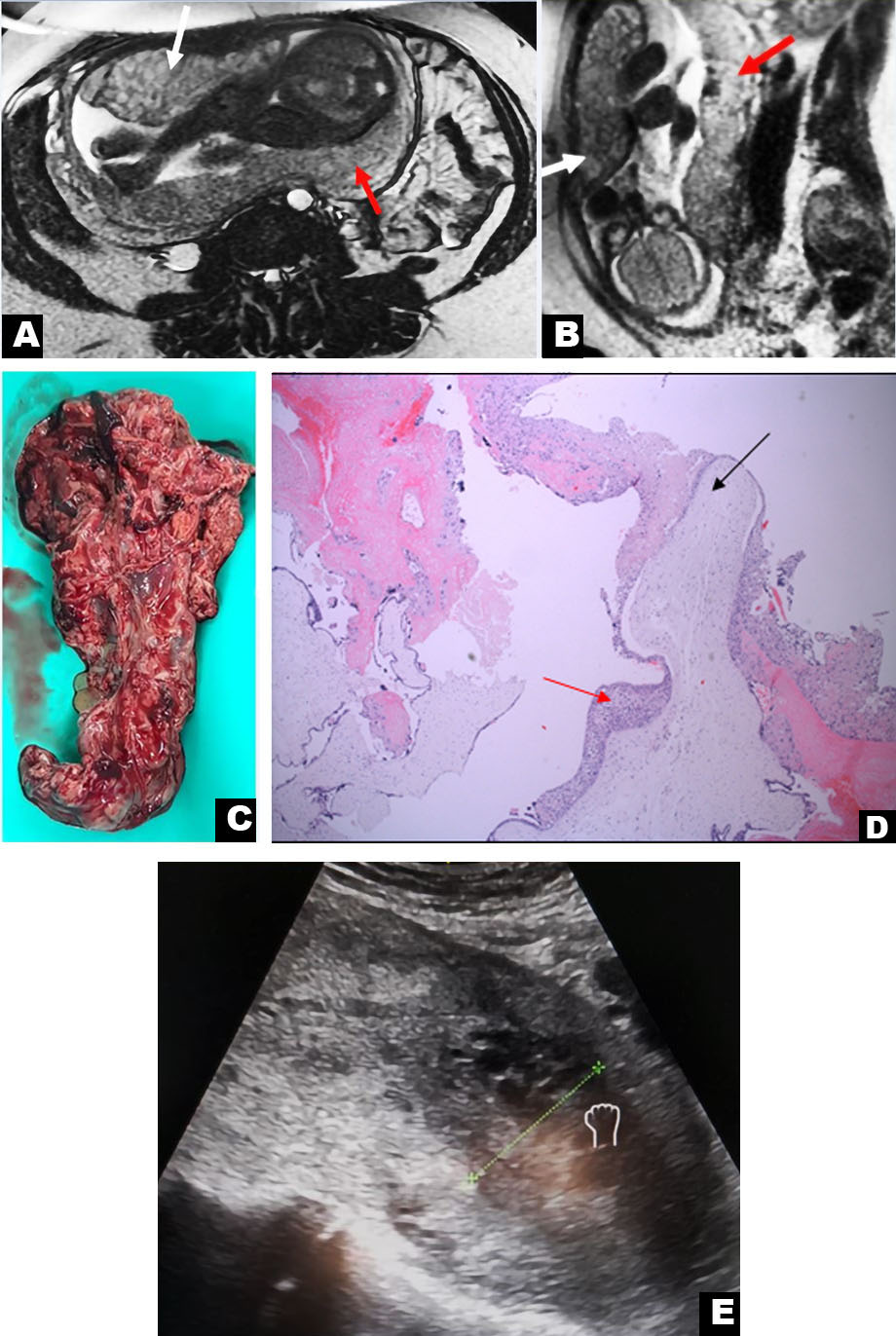
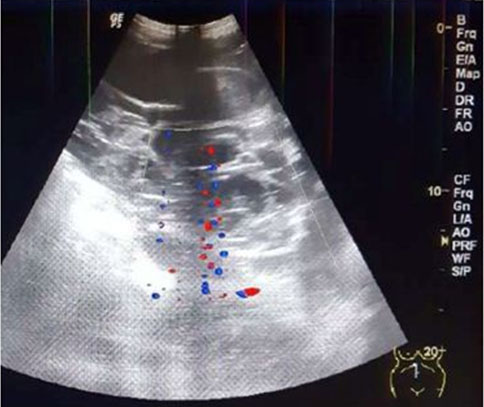
Obstetrical US at 22 weeks and 5 days gestational age for fetal survey revealed a single male fetus in vertex position with a posterior placenta free of the cervical os. Growth, fetal survey, and a biophysical profile were normal. An anterior mural based multi-cystic lenticular shaped uterine mass measuring 11.8 cm long × 4.2 cm AP × 12.8 cm W was completely separate from the posterior placenta in the gestational sac of the live fetus. Color Doppler evaluation showed prominent vascularity within the mass (Figure 1A and Figure 1B).
Magnetic resonance imaging at 26 weeks and 6 days gestational age showed that only one gestational sac was visible containing the live fetus, which was similar to the US findings. The multi-cystic mass measuring 13.1 cm L × 4.7 cm AP × 12.8 cm W was located between the myometrium and the amniotic sac (Figure 2A and Figure 2B). No evidence of hemorrhage was seen. The ovaries were normal, without evidence of theca lutein cysts.
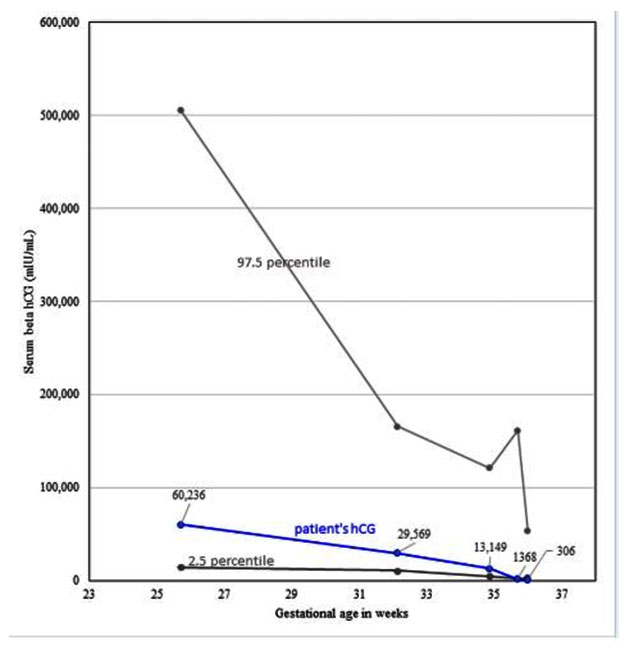
Because the serum beta hCG at 27 weeks 5 days was 60,239 mIU/mL (within normal limits for a singleton gestation at those dates [4]), a partial mole was suspected (Figure 3). Also considered in the differential diagnosis, but thought unlikely, was placental mesenchymal dysplasia, which may be associated with vaginal bleeding, increased vascularity in the mass, fetal intrauterine growth retardation, and sometimes with Beckwith-Wiedemann syndrome [7]. In contrast to molar pregnancy, mesenchymal dysplasia occurs within the placenta of the live fetus, not separately from it [8],[9].
The patient spontaneously delivered a live infant at 35 weeks and 4 days gestational age. The placenta and chorioamniotic membrane of the live fetus appeared normal on gross examination. Diagnosis of complete hydatidiform mole was confirmed by cytopathology without identifiable fetal tissue in the mass. The mass had chorionic villi demonstrating diffuse hydatidiform swelling [10] (Figure 2C, Figure 2D, Figure 2E).
Discussion
Gestational trophoblastic disease includes a broad spectrum of findings ranging from complete and partial hydatidiform mole to placental site trophoblastic tumor, chorioadenoma destruens, and choriocarcinoma. Complete hydatidiform mole with a coexisting live fetus is one of the rarest presentations of trophoblastic disease [10]. It is even more unusual with a normal hCG for gestational age.
Complete or partial hydatidiform moles are premalignant conditions that require mandatory evacuation of the uterus [1]. Management with a concomitant live fetus is even more complicated, as there are increased risks of vaginal bleeding, prematurity, intrauterine fetal demise, preeclampsia, hyperthyroidism, ovarian theca lutein cysts, uterine rupture, and malignant degeneration [3],[11],[12],[13].
Patients with molar pregnancies also have an increased risk of gestational trophoblastic disease in subsequent pregnancies. Patients with a complete mole and twin, as in this case, have a higher risk of gestational trophoblastic neoplasia as compared to patients with a single complete hydatidiform moles [14]. After evacuation of hydatidiform moles, serial measurements of hCG for several months after delivery is recommended to surveil for the development of gestational trophoblastic neoplasia [15].
With complete molar pregnancies, serum beta hCG usually exceeds 100,000 mIU/mL. However, with partial moles, serum beta hCG can be within the normal range. Therefore, patients with partial moles typically show less severe symptoms than with complete moles [16]. Since the present rare case had normal for gestational age hCG levels, the patient lacked symptoms that would be expected with a complete hydatidiform mole (i.e., no evidence of hyperemesis gravidarum, preeclampsia, nor hyperthyroidism [15]).
Ultrasound imaging in this case showed an indeterminate uterine mass separate from the placenta of the normal twin, but there was doubt about the etiology of this mass. The differential diagnosis included a residual blighted twin with hydropic degeneration, partial molar pregnancy, placental mesenchymal dysplasia in a succenturiate placenta (very rare) [5], or a subchorionic hemorrhage. Magnetic resonance imaging narrowed down the differential diagnosis by excluding hematoma and placental mesenchymal dysplasia. Partial hydatidiform mole and complete mole with live twin were the main considerations. Magnetic resonance imaging demonstrated lack of molar infiltration into the endometrium and a separate live twin that led to the conservative decision to continue the pregnancy [17].
Conclusion
This rare case highlights the utility of MRI after equivocal US for diagnosis and optimized conservative management of a hydatidiform complete mole with concomitant live twin. The clinical presentation was complicated by the normal maternal serum hCG, a rare finding that explains the lack of signs and symptoms usually expected with a complete mole. As MRI provided the necessary detailed information to arrive a fitting differential diagnosis, conservative management resulted in a live born healthy fetus in this case. Obstetrical MRI can be essential in the workup of selected cases with an indeterminate uterine masses in pregnancy like this one.
REFERENCES
1.
Sebire NJ, Foskett M, Paradinas FJ, et al. Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet 2002;359(9324):2165–6. [CrossRef]
[Pubmed]

2.
Zaidi SF, Moshiri M, Osman S, et al. Comprehensive imaging review of abnormalities of the placenta. Ultrasound Q 2016;32(1):25–42. [CrossRef]
[Pubmed]

3.
Vaisbuch E, Ben-Arie A, Dgani R, Perlman S, Sokolovsky N, Hagay Z. Twin pregnancy consisting of a complete hydatidiform mole and co-existent fetus: Report of two cases and review of literature. Gynecol Oncol 2005;98(1):19–23. [CrossRef]
[Pubmed]

4.
Korevaar TIM, Steegers EAP, de Rijke YB, et al. Reference ranges and determinants of total hCG levels during pregnancy: The Generation R Study. Eur J Epidemiol 2015;30(9):1057–66. [CrossRef]
[Pubmed]

5.
Jha P, Paroder V, Mar W, Horowtiz JM, Poder L. Multimodality imaging of placental masses: A pictorial review. Abdom Radiol (NY) 2016;41(12):2435–44. [CrossRef]
[Pubmed]

6.
Freis A, Elsässer M, Sohn C, Fluhr H. Twin pregnancy with one fetus and one complete mole – A case report. Geburtshilfe Frauenheilkd 2016;76(7):819–22. [CrossRef]
[Pubmed]

7.
Edelstam G, Karlsson C, Westgren M, Löwbeer C, Swahn ML. Human chorionic gonadotropin (hCG) during third trimester pregnancy. Scand J Clin Lab Invest 2007;67(5):519–25. [CrossRef]
[Pubmed]

8.
Ulker V, Aslan H, Gedikbasi A, Yararbas K, Yildirim G, Yavuz E. Placental mesenchymal dysplasia: A rare clinicopathologic entity confused with molar pregnancy. J Obstet Gynaecol 2013;33(3):246–9. [CrossRef]
[Pubmed]

9.
Himoto Y, Kido A, Minamiguchi S, et al. Prenatal differential diagnosis of complete hydatidiform mole with a twin live fetus and placental mesenchymal dysplasia by magnetic resonance imaging. J Obstet Gynaecol Res 2014;40(7):1894–900. [CrossRef]
[Pubmed]

10.
Lipi LB, Philp L, Goodman A. A challenging case of twin pregnancy with complete hydatidiform mole and co-existing normal live fetus – A case report and review of the literature. Gynecol Oncol Rep 2019;31:100519. [CrossRef]
[Pubmed]

11.
Sharon NZ, Maymon R, Melcer Y, Jauniaux E. Obstetric outcomes of twin pregnancies presenting with a complete hydatidiform mole and coexistent normal fetus: A systematic review and meta-analysis. BJOG 2020;127(12):1450–7. [CrossRef]
[Pubmed]

12.
Massardier J, Golfier F, Journet D, et al. Twin pregnancy with complete hydatidiform mole and coexistent fetus: Obstetrical and oncological outcomes in a series of 14 cases. Eur J Obstet Gynecol Reprod Biol 2009;143(2):84–7. [CrossRef]
[Pubmed]

13.
Sánchez-Ferrer ML, Hernández-Martínez F, Machado-Linde F, Ferri B, Carbonel P, Nieto-Diaz A. Uterine rupture in twin pregnancy with normal fetus and complete hydatidiform mole. Gynecol Obstet Invest 2014;77(2):127–33. [CrossRef]
[Pubmed]

14.
Lin LH, Maestá I, Braga A, et al. Multiple pregnancies with complete mole and coexisting normal fetus in North and South America: A retrospective multicenter cohort and literature review. Gynecol Oncol 2017;145(1):88–95. [CrossRef]
[Pubmed]

15.
Lurain JR. Gestational trophoblastic disease I: Epidemiology, pathology, clinical presentation, and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol 2010;203(6):531–9. [CrossRef]
[Pubmed]

16.
Cavaliere A, Ermito S, Dinatale A, Pedata R. Management of molar pregnancy. J Prenat Med 2009;3(1):15-7.
[Pubmed]

17.
Bajaj SK, Misra R, Gupta R, Nisha B, Thukral BB. Complete hydatidiform mole with coexisting twin fetus: Usefulness of MRI in management planning. J Obstet Gynaecol India 2014;64(Suppl 1):9–13. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Sarah Weinstein - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Yuval Ginsberg - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ari Reiss - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Marcia C Javitt - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Sarah Weinstein et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.