|
Case Report
What to learn from a case of short-term pregnancy after uterine artery embolization for acquired uterine arteriovenous malformation
1 Obstetrics and Gynecology Unit, Maurizio Bufalini Hospital, Ausl Romagna, Cesena, Italy
2 Radiology Unit, Maurizio Bufalini Hospital, Ausl Romagna, Cesena, Italy
Address correspondence to:
Serena Solfrini
M.D., Obstetrics and Gynecology Unit, Maurizio Bufalini Hospital, Ausl Romagna, Viale G. Ghirotti 286, Cesena (FC) 47521,
Italy
Message to Corresponding Author
Article ID: 100105Z08SS2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Solfrini S, Rossi M, Tarsitano F, Giampalma E, Antonazzo P. What to learn from a case of short-term pregnancy after uterine artery embolization for acquired uterine arteriovenous malformation. J Case Rep Images Obstet Gynecol 2022;8: 100105Z08SS2022.ABSTRACT
Introduction: Acquired uterine arteriovenous malformations are rare causes of bleeding occurring after uterine curettage, gestational disease, abortion, or delivery. Uterine artery embolization is a safe and effective procedure to treat bleeding, although outcomes on fertility and subsequent pregnancy are yet to be defined.
Case Report: A 34-year-old nulliparous woman underwent medical treatment with misoprostol for first trimester miscarriage. After two weeks a transvaginal ultrasound found a round myometrial lesion with hypoechoic spaces, abundant vascularization suggestive of an acquired uterine arteriovenous malformation. A conservative management was adopted with ultrasound follow-up. Twenty days later a severe bleeding occurred and the patient was treated with bilateral uterine artery embolization. After three months the patient got pregnant and gave birth at term to a healthy and normal weight baby. Third stage of labor was complicated by placental retention requiring manual removal and postpartum hemorrhage managed with uterine hemostatic balloon positioning and blood transfusion.
Conclusion: In this case uterine artery embolization was followed by a very short-term pregnancy with vaginal birth and excellent neonatal outcome. Uterine ischemic changes could predispose to placental adhesion anomalies and contraction dysfunctions. An accurate ultrasound screening for placenta accreta spectrum disorders and an active management of third stage could be suggested.
Keywords: Arteriovenous malformation, Placenta accreta, Pregnancy, Uterine artery embolization
Introduction
Acquired uterine arteriovenous malformations (UAVM) are abnormal vascular connections resulting from uterine surgery, trophoblastic disease, or miscarriage [1],[2]. Pelvic ultrasound is the first investigation for suspected cases, although angiography is the gold standard for diagnosis [3],[4]. Management options for UAVM include conservative follow-up for asymptomatic cases, medical treatment, uterine artery embolization (UAE), or hysterectomy in case of severe bleeding [1],[2],[5]. Uterine artery embolization is a safe and effective technique for resolution of bleeding due to UAVM allowing to preserve the uterus, although outcomes in terms of future fertility and pregnancy complications are still controversial [6],[7]. This case presents a spontaneous pregnancy occurred immediately after an emergency procedure of bilateral UAE for bleeding due to a UAVM.
Case Report
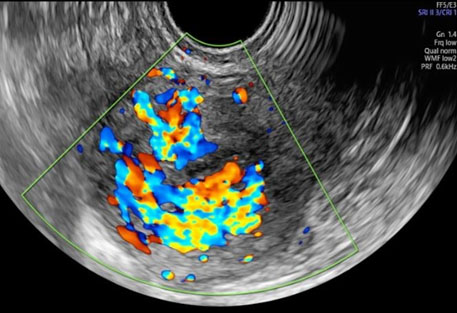
A 34-year-old nulliparous woman underwent medical treatment with misoprostol for an anembryonic pregnancy at nine weeks of amenorrhea. After two weeks a follow-up transvaginal ultrasound (TVUS) showed no retained products of conception (RPOC) with an endometrial thickness of 2.2 mm. It was found an interrupted endometrial-myometrial border in correspondence of a round myometrial lesion of 43×29×31 mm located in the posterior uterine wall (Figure 1). This myometrial lesion was described according to Morphological Uterus Sonographic Assessment (MUSA) terminology for gray scale and Doppler evaluation. Sonographic features showed in Figure 1 at gray scale were: undefined margin, non-uniform echogenicity with multiple irregular internal hypoechogenic spaces. Doppler images showed intralesional multiple vessels and a color score of 4 (Figure 2). The highest peak systolic velocity (PSV) was 31 cm/sec. Ultrasound was performed by an expert sonographer (S.S) with GE Voluson E8 equipment (GE Healthcare, Zipf, Austria) with vaginal probe frequencies of 4–7 MHz. Pulse repetition frequencies for color Doppler used were 600–900 MHz. The highest PSV was recorded with 1 mm window in multiple measurements. According to Italian national protocol on misoprostol use for miscarriage all patients undergo to a TVUS after two weeks to check treatment outcome and presence of RPOC. The β-human chorionic gonadotropin (hCG) value was 13 mIU/mL at time of first ultrasound examination.
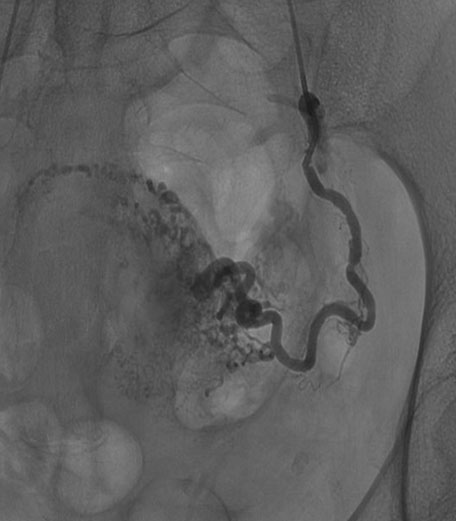
The case was discussed in a multidisciplinary meeting together with interventional radiologists. According to ultrasound features suggesting an acquired UAVM and to absence of bleeding, a conservative management with follow-up was adopted. Outpatient follow-up examination after two weeks was unvaried. Five days later the patient came to our emergency for heavy vaginal bleeding and the UAVM area was treated with bilateral UAE. Abdominal distal angiography in the early phase confirmed an arteriovenous shunt on posterior uterine supplied by both uterine arteries (Figure 3). Hyper selective bilateral UAE was successfully performed with Embozene Microspheres (CeloNova BioSciences, Inc., Texas, USA) showing the disappearance of the UAVM. Hemoglobin (Hb) was 11.5 g/dL at hospital admission and 8 g/dL after UAE. The patient was discharged after five days and no complications occurred. Transvaginal ultrasound after one month showed UAVM resolution. Three months later the patient got pregnant. Ultrasound showed placentation in the right posterior uterine wall. At 40 weeks the patient went into spontaneous labor and delivered a healthy baby of 3210 g with an Apgar score 9–10. The third stage of labor was complicated by postpartum hemorrhage (PPH) due to uterine atony and placental retention. Uterotonic treatment with oxitocin, sulprostone, and tranexamic acid was administered.
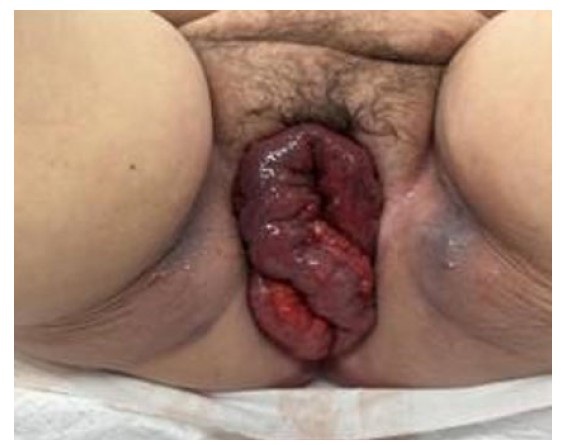
A manual removal of placenta was performed followed by Bakri Balloon positioning (Cook Medical, Inc., Indiana, USA). Placenta was intraoperative described as abnormally adherent to the posterior uterine wall. Estimated blood loss was 3000 mL. Two units of blood cells were transfused.
Patient’s informed consent has been obtained for scientific purpose.
Discussion
Acquired uterine arteriovenous malformations, also referred as enhanced myometrial vascularity (EMV), are abnormal vascular connections resulting mostly after uterine curettage, gestational trophoblastic disease, scar pregnancy or delivery [1],[2],[8]. Dysfunctions in vascular morphogenesis or in remodeling that do not regress spontaneously after pregnancy are at the origin of these lesions [1]. A 5.2% incidence of UAVM after dilatation and curettage has been reported, although the problem could be underestimated due to asymptomatic cases [1],[2],[3]. The reported incidence of EMV following first-trimester miscarriage is 1.52% [9]. Indeed, clinical presentation is variable from no symptoms or spotting to life-threatening uterine bleeding that requires emergency procedures for treatment. Although the gold standard for diagnosis remains angiography, TVUS scanning with color and spectral Doppler is the first diagnostic tool for suspected cases [3],[4],[5],[10]. Sonographic features mostly reported on TVUS are the presence of an inhomogeneous myometrial area located adjacent to the uterine cavity with hypo or an echogenic spaces showing an abundant and tortuous network of vessels at color Doppler. Spectral Doppler measurement demonstrates high velocity/low resistance blood flow with PSV > 20 cm/sec [1],[3],[4],[5],[9]. To the best of our knowledge this is the first case in which sonographic features of UAVM were described according to the standardized MUSA terminology with particular attention to endometrial-myometrial junction, margin and echogenicity of myometrial lesion, and color score [11]. We did not consider a diagnostic hysteroscopy for confirmation because an expert TVUS is able to diagnose a UAVM/EMV and to exclude the presence of RPOC in absence of an endometrial thickening and of any intracavitary echogenic vascularized area [3],[4],[5].
Management of UAVM/EMV should be individualized and influenced by the severity of symptoms. In mild or asymptomatic cases, a conservative management with follow-up is suggested; medical therapy with gonadotropin releasing hormone (GnRH) analogues, progestins, and methotrexate has also been described [2],[5],[9],[10],[12]. Ultrasound-guided dilatation and curettage is a safe option when RPOC are concomitant [5]. Moreover, a sonographic triage according to PSV value at diagnosis has been reported. Patients with higher PSV (>60 cm/sec) are more likely to undergo to surgical procedures (curettage, hysterectomy, or embolization) for uterine bleeding compared to cases with lower PSV which have more frequently a spontaneous resolution [4]. Considering the absence of RPOC and of bleeding symptoms we opted for a conservative management after a multidisciplinary discussion. In the case, despite the low PSV value, a severe uterine bleeding occurred and it was successfully resolved with bilateral UAE. Endovascular treatment with UAE is an effective and safe therapy to resolve heavy bleeding also due to UAVM; however, reported outcomes in terms of fertility and subsequent pregnancy are unclear [6],[7]. The rate of pregnancy after UAE for UAMV is 17% in a review but it is higher 85.7% in retrospective studies [1],[6]. Concerning pregnancy interval, a mean time period for subsequent pregnancy after UAVM treatment of 15.7 ± 11.7 and 19 ± 16.3 months is reported [2],[7]. In this case UAE was followed by a very short-term spontaneous pregnancy after only three-month interval after treatment. Moreover, UAE for PPH or uterine fibroids could be associated with uterine necrosis, spontaneous abortion, preterm birth, fetal growth restriction, and fetal death [6],[13]. Neonatal outcomes of pregnancy here described were excellent. Considering maternal morbidity, a major PPH that was resistant to uterotonic treatment and requiring manual removal of placenta occurred. According to the classification of placenta accreta spectrum disorders (PAS), an abnormally adherent placenta is clinically defined at vaginal delivery by “manual removal of placenta resulting in heavy bleeding requiring mechanical or surgical procedures” [14].
It has been reported that UAE is a major risk factor for PAS in subsequent pregnancies [13]. This finding might be related to ischemic phenomena which may occur after UAE. A reduced blood flow to the myometrium or damage to the endometrial-myometrial interface might potentially lead to placental adhesion anomalies. Moreover, a reduce uterine contraction in treated area could predispose to uterine atony. There is an ongoing debate on whether placentation disorders may be the consequence of surgical or radiological treatment for UAVM or whether UAVM arising on a damaged junction could predispose itself to PAS.
Conclusion
Acquired uterine arteriovenous malformations are rare but potentially life-threatening disease related to pregnancy even in absence of uterine surgical procedures. A transvaginal ultrasound in a first trimester outpatient unit could be useful after medical treatment of miscarriage or termination of pregnancy to early diagnose RPOC or UAVM/EVM. Diagnostic criteria of UAVM/EMV are not well defined, but a high index of suspicious is recommended when ultrasound features of a myometrial lesion like a “fire ball” appears at color Doppler. Sonographic description of these myometrial lesions according to MUSA consensus terminology could standardize imaging report and diagnosis. Management option in case of mild symptoms should be conservative with serial ultrasound follow-up. However, when a severe metrorrhagia occurs, UAE allows fertility preservation and bleeding resolution. Counseling for a spontaneous pregnancy after UAE for UAMV should be considered in women desiring fertility, despite the best interval to prevent complication has not been determinate. This case underlines the importance to look after these women as high-risk for placenta related complications, according to the reported association of UAE and PAS. An accurate ultrasound screening of placental anomalies should be suggested. Moreover, due to hemorrhagic risk, delivery should be planned in specialized obstetrics units, considering an active management of third stage of labor in order to prevent PPH.
REFERENCES
1.
2.
Peitsidis P, Manolakos E, Tsekoura V, Kreienberg R, Schwentner L. Uterine arteriovenous malformations induced after diagnostic curettage: A systematic review. Arch Gynecol Obstet 2011;284(5):1137–51. [CrossRef]
[Pubmed]

3.
Timmerman D, Wauters J, Van Calenbergh S, et al. Color Doppler imaging is a valuable tool for the diagnosis and management of uterine vascular malformations. Ultrasound Obstet Gynecol 2003;21(6):570–7. [CrossRef]
[Pubmed]

4.
Timor-Tritsch IE, Haynes MC, Monteagudo A, Khatib N, Kovács S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity/arteriovenous malformations. Am J Obstet Gynecol 2016;214(6):731.e1–731.e10. [CrossRef]
[Pubmed]

5.
Groszmann YS, Healy Murphy AL, Benacerraf BR. Diagnosis and management of patients with enhanced myometrial vascularity associated with retained products of conception. Ultrasound Obstet Gynecol 2018;52(3):396–9. [CrossRef]
[Pubmed]

6.
Delplanque S, Le Lous M, Proisy M, et al. Fertility, pregnancy, and clinical outcomes after uterine arteriovenous malformation management. J Minim Invasive Gynecol 2019;26(1):153–61. [CrossRef]
[Pubmed]

7.
Eling R, Kent A, Robertson M. Pregnancy after uterine arteriovenous malformation-case series and literature review. Australas J Ultrasound Med 2012;15(3):87–96. [CrossRef]
[Pubmed]

8.
Touhami O, Gregoire J, Noel P, Trinh XB, Plante M. Uterine arteriovenous malformations following gestational trophoblastic neoplasia: A systematic review. Eur J Obstet Gynecol Reprod Biol 2014;181:54–9. [CrossRef]
[Pubmed]

9.
Grewal K, Al-Memar M, Fourie H, Stalder C, Timmerman D, Bourne T. Natural history of pregnancy-related enhanced myometrial vascularity following miscarriage. Ultrasound Obstet Gynecol 2020;55(5):676–82. [CrossRef]
[Pubmed]

10.
Timmerman D, Van den Bosch T, Peeraer K, et al. Vascular malformations in the uterus: Ultrasonographic diagnosis and conservative management. Eur J Obstet Gynecol Reprod Biol 2000;92(1):171–8. [CrossRef]
[Pubmed]

11.
Van den Bosch T, Dueholm M, Leone FPG, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol 2015;46(3):284–98. [CrossRef]
[Pubmed]

12.
Degani S, Leibovitz Z, Shapiro I, Ohel G. Expectant management of pregnancy-related high-velocity uterine arteriovenous shunt diagnosed after abortion. Int J Gynaecol Obstet 2009;106(1):46–9. [CrossRef]
[Pubmed]

13.
Jitsumori M, Matsuzaki S, Endo M, et al. Obstetric outcomes of pregnancy after uterine artery embolization. Int J Womens Health 2020;12:151–8. [CrossRef]
[Pubmed]

14.
Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, Fox KA, Collins S; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet 2019;146(1):20–4. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Serena Solfrini - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Martina Rossi - Conception of the work, Design of the work, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Francesco Tarsitano - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Emanuela Giampalma - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Patrizio Antonazzo - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Serena Solfrini et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.